|
Module Overview
On April 26, 1986, two explosions destroyed Reactor 4 at the Chernobyl nuclear power plant in the Ukraine (formerly part of the Soviet Union). As a result, the reactor’s radioactive fuel, including the Cesium-137 isotope, spread across the surrounding environment and into the atmosphere.
Humans exposed to Cesium-137 and other radioactive materials risk significant impairments in health, including a variety of cancers, blood disorders, and other potentially fatal conditions. These risks are compounded by the fact that many radioactive materials linger in the environment for long periods of time, decaying only at very slow rates. For example, the half-life (the amount of time required for the material to decay by one-half of the original amount) of Cesium-137 is thirty years. Thus, a portion of the Cesium-137 released into the environment at Chernobyl is still there.

Radioactive decay and the concept of half-life are examples of exponential decay, functions that describe this type of behavior over time. In fact, in these functions the variable is time, and it is the exponent or power applied to a constant base. For Cesium-137, one form of the decay function is:
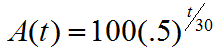
(where A is the amount of Cesium-137 remaining t years after the placement of 100 kilograms of the material).
The graph of this decay function is:
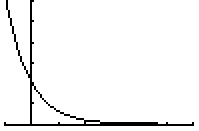
How could you determine the number of years, t, which must elapse before there is less than 1 kilogram of Cesium-137 remaining?
To answer this question, and many other such real-world problems, we will now investigate exponential and logarithmic functions. In this module, you will extend your knowledge of algebraic functions and learn how these functions help us understand many real-world phenomena.
|