Module 8: Thermochemistry
Calorimetry Scientific Investigation
![]()
![]() Before you begin the scientific investigation below, make sure to download the Calorimetry Scientific Investigation Report. As you complete this scientific investigation, fill in any needed information on the report template. If you need more information about each section of the report, please visit the Developmental module.
Before you begin the scientific investigation below, make sure to download the Calorimetry Scientific Investigation Report. As you complete this scientific investigation, fill in any needed information on the report template. If you need more information about each section of the report, please visit the Developmental module.
This scientific investigation is available below or in a printable version.
Introduction
As you learned in this topic, one use of calorimetry is to determine the amount of energy stored in the food items you eat. In this laboratory activity, you will investigate the amount of stored energy in a peanut. By completing this investigation, you will determine how many calories a peanut contains.
Please Note:
If you have a peanut allergy, please contact your instructor to receive information about an alternate assignment.
Objectives
In this scientific investigation, you will:
- measure the amount of energy stored in a peanut
Hypothesis
Using the Procedure and Data Collection section below, read through the procedural information for this scientific investigation. Based on your understanding of the procedure, develop your own hypotheses which describe your expected results. You should consider the following questions: How many calories do you expect one peanut to contain? How will you test the energy content of a peanut? Record these hypotheses in the Hypothesis section of your Calorimetry Scientific Investigation Report.
Equipment and Materials
- Goggles
- Apron
- A peanut
- A balance
- A cork
- A straight pin
- 50 mL of water
- A beaker or flask
- Ring stand
- Wire gauze
- Thermometer clamp
- Thermometer
- Lighter
- Ring and clamp
Procedure and Set-Up
Set-Up
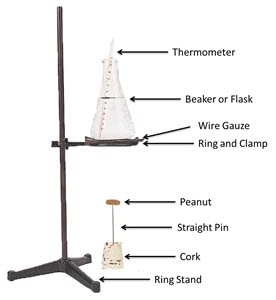 Position the equipment on the ring stand to match what is shown in the diagram to the right.
Position the equipment on the ring stand to match what is shown in the diagram to the right.
Important: Make sure to exercise caution when completing this experiment. Follow proper laboratory etiquette by wearing safety goggles and an apron throughout this experiment.
Procedure
- Determine the mass of the peanut in grams. Record the mass in the data table provided.
- Pour 50 mL of water into the beaker or flask.
- Record the initial temperature of the water in the data table provided.
- Carefully place the peanut on the straight pin.
- Using caution, ignite the peanut with the lighter or matches.
- Allow the remains of the peanut to cool and then record its final mass in grams in the data table provided..
- Calculate the amount of energy in calories of the peanut, using the following equation.
Cal= mwater x ΔHwater x 1.0 cal/g°C
Data
Record any observations and data you had while you conducted the experiment on the data table provided in the Data section of your Calorimetry Scientific Investigation Report.
Data Analysis
In the Data Analysis section of your Calorimetry Scientific Investigation Report, provide the responses to the following questions:
- What happened to the peanut? Explain.
- What happened to the water? Explain.
- What must happen to the food you eat before your cells can use that energy?
Conclusion
Using the Conclusion section of your Calorimetry Scientific Investigation Report, compose three to four sentences describing an overall conclusion about the results of the experiment. Base your conclusions on your data. Were your hypotheses true or false, how do you know? Use the data and notes that you collected from your experiment to form your conclusion. Make sure that your include information that your gained from data analysis to support your conclusion.
Experimental Sources of Error
On your Calorimetry Scientific Investigation Report, provide responses to the following questions: Are there any sources of error? If so, what are they, and what could be done to minimize error?
![]() Once you have completed the Calorimetry Scientific Investigation Report, please submit your work to the dropbox.
Once you have completed the Calorimetry Scientific Investigation Report, please submit your work to the dropbox.
![]()
Once you have completed this module, please complete the module test.