
Separation Techniques
Part A: Separation Procedure
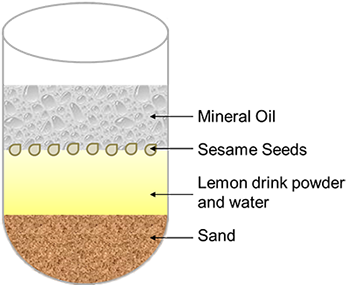 Part of performing a successful separation of a mixture in the lab is knowing the order in which to perform the different techniques. Now that you have studied the various types of separation techniques, it is time to determine how to separate a mixture of lemon drink powder, mineral oil, sesame seeds, sand, and water into its five component parts. Look carefully at the mixture to the right and make not of which substance is in which region. Using your knowledge of separation techniques, write a step by step procedure that will allow you to separate each of the five parts. Make sure to include information about the needed equipment, as well as any information about the physical properties of the components that would allow you to separate them in the manner in which you suggest.
Part of performing a successful separation of a mixture in the lab is knowing the order in which to perform the different techniques. Now that you have studied the various types of separation techniques, it is time to determine how to separate a mixture of lemon drink powder, mineral oil, sesame seeds, sand, and water into its five component parts. Look carefully at the mixture to the right and make not of which substance is in which region. Using your knowledge of separation techniques, write a step by step procedure that will allow you to separate each of the five parts. Make sure to include information about the needed equipment, as well as any information about the physical properties of the components that would allow you to separate them in the manner in which you suggest.
![]()
Once you have completed your separation techniques procedure design, please submit your work to the dropbox.
Part B: Chromatography Scientific Investigation

![]() Before you begin the scientific investigation below, make sure to download the Chromatography Scientific Investigation Report. As you complete this scientific investigation, fill in any needed information on the report template. If you need more information about each section of the report, please visit the Developmental Module.
Before you begin the scientific investigation below, make sure to download the Chromatography Scientific Investigation Report. As you complete this scientific investigation, fill in any needed information on the report template. If you need more information about each section of the report, please visit the Developmental Module.
This scientific investigation is available below or in a printable version.
Introduction
Paper chromatography is a technique that involves placing a small dot or line of sample solution onto a strip of chromatography paper. The paper is then placed in a jar containing a small layer of solvent and is sealed. As the solvent rises through the paper, it meets the sample mixture, which then travels up the paper with the solvent. If the components of the mixture are soluble in the solvent being used, the mixture will be carried up the paper strip as the solvent travels. Mixtures contain substances with a variety of different solubility. The more soluble substances move faster and to a greater distance than those that are less soluble.
Objectives
In this scientific investigation, you will:
- complete the chromatography technique on four different groups;
- compare the length of travel for water and ink during chromatography; and
- analyze the mixtures of ink found in markers.
Hypothesis
Using the Procedure and Data Collection section below, read through the procedural information for this scientific investigation. Based on your understanding of the procedure, develop your own hypotheses which describe your expected results. Specifically, how do you think the ink of each marker will behave when chromatography is performed? Record these hypotheses in the Hypothesis section of your Chromatography Scientific Investigation Report.
Equipment and Materials
- 1 large beaker
- 1 small beaker filled with water
- 4 pieces of filter paper or a coffee filter cut into four even strips
- 4 different black markers for testing
- Permanent marker
- Timer
- Masking tape
Procedure and Data Collection
- Label each marker with a piece of masking tape numbered one through four.
- Label each piece of filter paper with a number matching it with one of the marker numbers.
- Using one of the testing markers, draw a thick line horizontally across the matching filter paper about 1/4th inch from the bottom.
- Pour a small amount of water into the large beaker and then dip the end of the filter paper in the water. Make sure that the ink line does not touch the water.
- Allow the water to move up the paper for five minutes. Remove the strip and using masking tape, hang the paper on the side of a table.
- Repeat steps 3 through 5 for each of the marker and filter paper combinations and wait at least five minutes after the final marker has been dipped.
- Once all of the maker and filter paper combinations are hanging from the table, complete the second two columns of the data table in the Data section of your Chromatography Scientific Investigation Report. For each ink, measure the distance (in millimeters) that the water traveled, as well as the distance (in millimeters) from the ink's starting point.
- Complete the final column of the data table in the Data section of your Chromatography Scientific Investigation Report by making a sketch of each of the filter papers.
Data
Use the area provided on your Chromatography Scientific Investigation Report to record your data from this scientific investigation, as well as make sketches of your filter papers. The data table is also shown below:
| Sample Number | Distance Water Traveled |
Distance Ink Traveled |
Sketch of Filter Paper |
| 1 | |||
| 2 | |||
| 3 | |||
| 4 |
Data Analysis
In the Data Analysis section of your Chromatography Scientific Investigation Report, provide responses to the following questions:
- What is the solvent in this scientific investigation?
- Are all of the inks the same? If not, how are they different?
- Do you think these inks are homogeneous mixtures or heterogeneous mixtures?
- Based on what you have learned about chromatography, what causes the inks to separate?
Conclusion
Using the Conclusion section of your Chromatography Scientific Investigation Report, compose three to four sentences describing an overall conclusion based on your data. Were your hypotheses true or false, and how do you know? Use the data and notes that you collected from your investigation to form your conclusion. Make sure that you include information that you gained from data analysis to support your conclusion.
Experimental Sources of Error
On your Chromatography Scientific Investigation Report, provide responses to the following questions: Are there any sources of error? If so, what are they, and what could be done to minimize error?
![]() Once you have completed your Chromatography Scientific Investigation, please submit your work to the dropbox.
Once you have completed your Chromatography Scientific Investigation, please submit your work to the dropbox.



