
Neutralization Reactions
 On the pH scale, any substance that has a pH of seven is considered neutral. Acids have a low pH and bases have a high pH. What happens when an acid and a base react with each other? Since acids have a low pH and bases have a high pH, the two will neutralize.
On the pH scale, any substance that has a pH of seven is considered neutral. Acids have a low pH and bases have a high pH. What happens when an acid and a base react with each other? Since acids have a low pH and bases have a high pH, the two will neutralize.
Imagine for a moment that you spill a strong acid, such as nitric acid, on a laboratory bench. Since nitric acid causes chemical burns, how would you clean it up? The best method involves pouring a basic solution on the acid spill. This neutralizes that acid and allows the spill to be completely wiped up. Neutralization is not just important if correcting laboratory mishaps. Neutralization reactions help you maintain a balance of chemicals in your body. These types of reactions also help the oceans and lakes to remain in chemical balance. Neutralization reactions are used to produce a wide variety of products from fertilizers to pharmaceuticals.
What occurs during a neutralization reaction? A neutralization reaction is a reaction in which both an acid and a base react in an aqueous solution to produce a salt and water. The type of sodium chloride that is produced in neutralization reactions is called a salt. However, do not confuse all of these salts as table salt. Table salt forms from a different reaction. Salts are ionic compounds that are composed of a cation base and an anion acid. By definition, a salt is a neutral ionic compound.
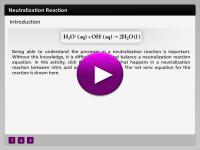 Neutralization reactions are classified into two categories. Strong acids will require a strong base to neutralize. But what happens when a weak acid reactions with a strong base? In this activity, click on the steps to observe what happens in a neutralization reaction between nitric acid and sodium hydroxide.
Neutralization reactions are classified into two categories. Strong acids will require a strong base to neutralize. But what happens when a weak acid reactions with a strong base? In this activity, click on the steps to observe what happens in a neutralization reaction between nitric acid and sodium hydroxide.
View a printable version of the interactivity.
Writing the Equation and Predicting the Products of a Neutralization Reaction
 Writing the equations for acid and base reactions is similar to writing the equations for a double replacement reaction. The process involves predicting the formulas for the products. However, if the product is either carbonate or hydrogen carbonate the reaction is written differently. View this presentation to learn how to predict the products and write a neutralization reaction.
Writing the equations for acid and base reactions is similar to writing the equations for a double replacement reaction. The process involves predicting the formulas for the products. However, if the product is either carbonate or hydrogen carbonate the reaction is written differently. View this presentation to learn how to predict the products and write a neutralization reaction.
View a printable version of the interactivity.


