Le Chatelier's Principle and Effects on Equilibrium

![]() Before you begin the scientific investigation below, make sure to download the Le Chatelier’s Principle Scientific Investigation Report. As you complete this scientific investigation, fill in any needed information on the report template. If you need more information about each section of the report, please visit the Developmental Module.
Before you begin the scientific investigation below, make sure to download the Le Chatelier’s Principle Scientific Investigation Report. As you complete this scientific investigation, fill in any needed information on the report template. If you need more information about each section of the report, please visit the Developmental Module.
This scientific investigation is available below or in a printable version.
Introduction
Le Chatelier’s Principle states that if a system at equilibrium is subjected to a stress, the equilibrium is shifted in the direction that tends to relieve the stress. This shift will cause the reaction to reach a new equilibrium. Le Chatelier’s Principle is an example of cause and effect. In this investigation, you will observe Le Chatelier’s Principle as you manipulate one stress: temperature.
Objectives
In this scientific investigation, you will:
- observe the effects of temperature on a reversible reaction; and
- observe how the concentrations of the products and reactants change as the reaction reaches equilibrium.
Hypothesis
Using the Procedure and Data Collection section below, read through the procedural information for this scientific investigation. Based on your understanding of the procedure, develop your own hypotheses which describe the expected results. You should consider the following questions: How will temperature affect the equilibrium of a reversible reaction? How will the concentrations of reactants change as equilibrium is reached? Record these hypotheses in the Hypothesis section of your Le Chatelier’s Principle Scientific Investigation Report.
Required Simulation
Reactions and Rates Simulation
(click on image below to run simulation)
Provided by:
PhET Interactive Simulations
University of Colorado
http://phet.colorado.edu
Procedure and Data Collection
Simulation Set-Up
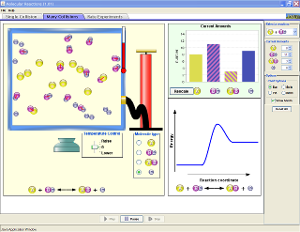
 Open the Reactions and Rates simulation.
Open the Reactions and Rates simulation.- Click on the Many Collisions tab. Click Bar under Chart Options.
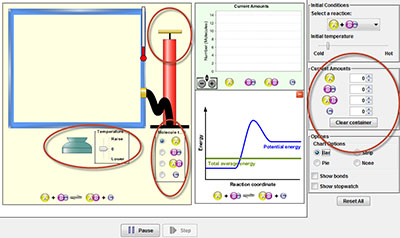
- Take a few moments to become familiar with the simulator. Add amounts of reactants, change the temperature, and pump the handle. View the image to the right to see where each of these controls are located.
- Once you are done experimenting with the simulation, click Reset All to reset the simulation.
Procedures
- Click on the Many Collisions tab. Click Bar under Chart Options.
- Under Current Amounts, give both A and Bc an amount of 25.
- Using the temperature menu, raise the temperature until the experiment reaches equilibrium. Equilibrium is indicated by the Reaction Coordinate graph to the right of the container.
- Record the amounts of reactants and products in the data table provided on your Le Chatelier’s Principle Scientific Investigation Report.
- Repeat Steps 1-4 four times to ensure a valid experiment through repeated trials.
Data
Use the data table provided in the Data section of your Le Chatelier’s Principle Scientific Investigation Report to record your data from this scientific investigation. The data table is also shown below.
Current Amounts |
Trial #1 |
Trial #2 |
Trial #3 |
Trial #4 |
|---|---|---|---|---|
| A | ||||
| Bc | ||||
| AB | ||||
| c |
Data Analysis
In the Data Analysis section of your Le Chatelier’s Principle Scientific Investigation Report, provide the answers to the following questions:
- How did the concentrations of the reactants and products change as the reaction came to equilibrium?
- Explain how Le Chatelier’s Principle is an example of cause and effect. What are some causes? What are some effects?
Conclusion
In the Conclusion section of your Le Chatelier’s Principle Scientific Investigation Report, compose three to four sentences describing an overall conclusion about the Le Chatelier’s Principle. Base your conclusions on your data. Were your hypotheses true or false, and how do you know? Use the data and notes that you collected from your simulation experience to form your conclusion. Make sure that your include information that your gained from data analysis to support your conclusion.
Experimental Sources of Error
On your Le Chatelier’s Principle Scientific Investigation Report, provide responses to the following questions: Are there any sources of error? If so, what are they, and what could be done to minimize error?
![]() Once you have completed the Le Chatelier’s Principle Scientific Investigation Report, please submit your work to the dropbox.
Once you have completed the Le Chatelier’s Principle Scientific Investigation Report, please submit your work to the dropbox.