
Foundations of Atomic Structure - Democritus and Dalton
Foundations of Atomic Structure
 For centuries, people wondered about the tiniest particle of matter that could exist. Over time, different philosophers and scientists contributed to the theory of the atom. Using the arrows in the upper right corner of the activity, view an interactive timeline of the contributions made by these philosophers and scientists.
For centuries, people wondered about the tiniest particle of matter that could exist. Over time, different philosophers and scientists contributed to the theory of the atom. Using the arrows in the upper right corner of the activity, view an interactive timeline of the contributions made by these philosophers and scientists.
View a printable version of the interactivity.
John Dalton's Atomic Theory and Chemical Formulas
John Dalton's Atomic Theory had four main points:
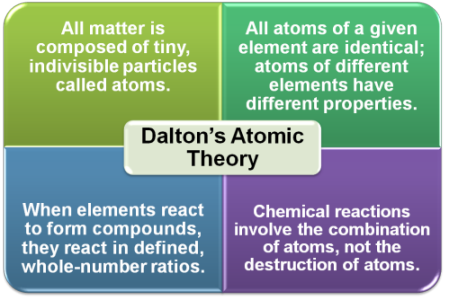
 John Dalton asserted that atoms were the smallest building blocks of matter and could not be subdivided, created, or destroyed into smaller particles. He assumed that the appearance of atoms was similar to the image shown to the left. Dalton’s theory was able to explain many important observations that other scientists had made, like the Law of Constant Composition. This law states that compounds always contain the same type and number of atoms. In other words, a given compound will always contain the same proportion by mass of its particular elements. Dalton’s atomic theory was used to predict how a pair of elements would combine to form more than one compound. For example, sulfur and oxygen might form a compound where there is one sulfur atom combined with each oxygen atom (written SO), or the atoms might combine in a compound where there are two oxygen atoms combine with each sulfur atom (written SO2).
John Dalton asserted that atoms were the smallest building blocks of matter and could not be subdivided, created, or destroyed into smaller particles. He assumed that the appearance of atoms was similar to the image shown to the left. Dalton’s theory was able to explain many important observations that other scientists had made, like the Law of Constant Composition. This law states that compounds always contain the same type and number of atoms. In other words, a given compound will always contain the same proportion by mass of its particular elements. Dalton’s atomic theory was used to predict how a pair of elements would combine to form more than one compound. For example, sulfur and oxygen might form a compound where there is one sulfur atom combined with each oxygen atom (written SO), or the atoms might combine in a compound where there are two oxygen atoms combine with each sulfur atom (written SO2).
Writing Chemical Formulas
 Now that you understand the basics of the Law of Constant Composition, view this presentation to learn more about writing chemical formulas. Make sure to practice your formula writing skills by completing the activity at the end of the presentation.
Now that you understand the basics of the Law of Constant Composition, view this presentation to learn more about writing chemical formulas. Make sure to practice your formula writing skills by completing the activity at the end of the presentation.
View a printable version of the interactivity.
Foundations of Atomic Structure Review

![]() After exploring the foundations of atomic structure and writing a chemical formulas, it is time to review your knowledge in this non-graded activity. Read the set of directions associated with each question, provide your answer(s), and click SUBMIT to check your response(s). Click on the interactivity thumbnail, and then click NEXT to get started.
After exploring the foundations of atomic structure and writing a chemical formulas, it is time to review your knowledge in this non-graded activity. Read the set of directions associated with each question, provide your answer(s), and click SUBMIT to check your response(s). Click on the interactivity thumbnail, and then click NEXT to get started.


