
The Nucleus
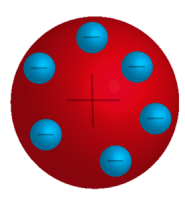
The Plum Pudding Model
At the beginning of the 20th century, scientists knew very little about the atom except that it contained negative particles called electrons. They knew that matter must be electrically neutral, so they came to accept that the Plum Pudding Model of the atom was correct. This model asserted that the atom was a positively-charged sphere with electrons scattered throughout.
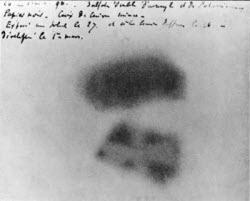
Becquerel's Photographic Plate
Around that time, the French physicist Henri Becquerel made an interesting discovery in a French lab. Becquerel noticed that when certain uranium salts were left on photographic plates in the dark, they became exposed. In 1896, he declared that he had accidentally discovered a new penetrating ray called “radioactivity”. His students, Marie and Pierre Curie, also performed experiments that showed that other elements emitted radioactivity. In 1903, a French physicist named Wilhelm Roentgen seemed to discover mysterious rays he called “x-rays.” The Curies and Becquerel shared the Nobel Prize in Physics for their discovery in 1903. It was a very exciting time in the development of the study of atomic structure. Unfortunately, Becquerel did not live long enough to see how important his discovery would become to this development. He developed serious burns on his skin from handling radioactive stones and died suddenly at the age of 55, only twelve years after his discovery.
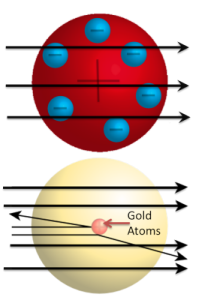
Model of the Gold Foil Experiment
It was not very long before a brilliant scientist, Ernest Rutherford, began studying radioactivity and discovered two important emissions from radioactive atoms. He named them alpha and beta rays. His work led to the understanding that when heavy atoms decay into lighter atoms, they emit alpha and beta rays. The discovery of these particles led to his famous “Gold Foil Experiment”.
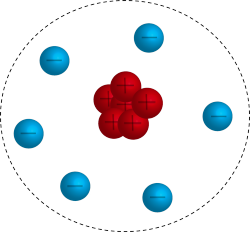
Rutherford's Atomic Model
In Rutherford's Gold Foil Experiment (shown in the image to the left), positive particles in alpha rays were directed towards gold atoms and then observed as they passed through the atoms. A very small percentage of the positively charged alpha particles were deflected by something with a like charge, making the structure of the atom similar to the image shown to the right. Rutherford’s Gold Foil Experiment proved two very important things about the atom:
- The atom is mostly empty space. Most of the alpha particles shot straight through the gold foil with nothing to stop them.
- The atom has a very small and dense positively charged center, which is now known to be the nucleus.
![]() Was Ernest Rutherford a wizard, or simply the first true alchemist? To review how the atomic structure was discovered, please view the video clip 1897-First Subatomic Particle Found: The Electron from eMediaVA℠.
Was Ernest Rutherford a wizard, or simply the first true alchemist? To review how the atomic structure was discovered, please view the video clip 1897-First Subatomic Particle Found: The Electron from eMediaVA℠.
Finding the Nucleus Review

![]() Amazing advancements in science took place with the discoveries of Becquerel, the Curies, and Rutherford. A better model of the atom was constructed because of their efforts. In this non-graded activity, read each statement and decide whether it is True or False. Then, select the appropriate answer and click SUBMIT to check your response. Click on the interactivity thumbnail, and then click NEXT to get started.
Amazing advancements in science took place with the discoveries of Becquerel, the Curies, and Rutherford. A better model of the atom was constructed because of their efforts. In this non-graded activity, read each statement and decide whether it is True or False. Then, select the appropriate answer and click SUBMIT to check your response. Click on the interactivity thumbnail, and then click NEXT to get started.


