
Nuclear Chemistry
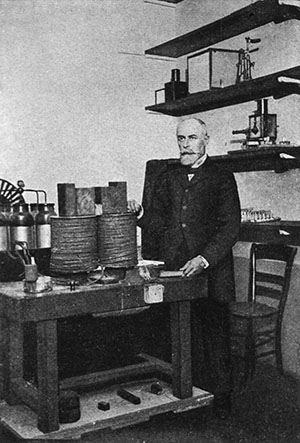
Henri Becquerel in his laboratory
The story of nuclear discovery begins with Henri Becquerel. It was in 1896 that he made the surprise discovery of radioactivity when he found that a photographic plate had become exposed when uranium ore was left on it. This happened even with the complete absence of light. He concluded that the uranium had some type of spontaneous emission. Pierre and Marie Curie became very interested in these emissions, which they called “radioactivity”. Ernest Rutherford added to the knowledge about radioactivity when he used radioactive emissions to determine more about the structure of the atom, including the nucleus. He called the individual rays emitted by radioactivity alpha, beta and gamma rays. With these three discoveries, scientists had finally achieved what the alchemists had been trying to do for hundreds of years. When alpha, beta, or gamma rays are emitted from the nucleus of a radioactive atom, they are changed into another element.
What is radioactivity? You might know radioactivity as radioactive decay or nuclear decay. All of these terms describe the process by which a nucleus of an unstable atom loses energy by emitting particles of ionizing radiation. Any material that emits ionizing radiation in the form of alpha particles, beta particles, and gamma rays is considered radioactive.
Three Types of Radiation
 Once an isotope becomes unstable, it can emit three different types of radiation. In each of the three cases, the parent (original) material becomes radioactive to start the decay process. In both alpha and beta decay, a daughter (new) material is created as a result. In this interactivity, click on each of the plus sign markers to learn more about the three different types of radiation.
Once an isotope becomes unstable, it can emit three different types of radiation. In each of the three cases, the parent (original) material becomes radioactive to start the decay process. In both alpha and beta decay, a daughter (new) material is created as a result. In this interactivity, click on each of the plus sign markers to learn more about the three different types of radiation.
View a printable version of the interactivity.
Half-Life
 Every isotope takes a certain amount of time to turn into a new element. Each element has a unique rate of decay. Some elements take a very long time to decay; others decay within fractions of a second. Scientists use half-life to measure the rate of decay in radioactive isotopes. Half-life is the amount of time it takes for half of the sample to decay. In this interactivity, click on each of the place markers to learn more about the concept of half-life.
Every isotope takes a certain amount of time to turn into a new element. Each element has a unique rate of decay. Some elements take a very long time to decay; others decay within fractions of a second. Scientists use half-life to measure the rate of decay in radioactive isotopes. Half-life is the amount of time it takes for half of the sample to decay. In this interactivity, click on each of the place markers to learn more about the concept of half-life.
View a printable version of the interactivity.
Nuclear Processes
 Nuclear fusion and nuclear fission are two different reactions in which energy is released from the bonds between the particles within the nucleus. The main difference between these two processes is that fission is the splitting of an atom into two or more smaller ones, while fusion is the fusing of two or more smaller atoms into a larger one. In this interactivity, click on each of the tabs to investigate the processes of nuclear fusion and nuclear fission in more detail.
Nuclear fusion and nuclear fission are two different reactions in which energy is released from the bonds between the particles within the nucleus. The main difference between these two processes is that fission is the splitting of an atom into two or more smaller ones, while fusion is the fusing of two or more smaller atoms into a larger one. In this interactivity, click on each of the tabs to investigate the processes of nuclear fusion and nuclear fission in more detail.
View a printable version of the interactivity.
Nuclear Chemistry Review

![]() Now that you have explored nuclear chemistry, complete this non-graded activity to review your knowledge. Read the directions associated with each question and provide your response. Then, click SUBMIT to check your answers. Click NEXT to get started.
Now that you have explored nuclear chemistry, complete this non-graded activity to review your knowledge. Read the directions associated with each question and provide your response. Then, click SUBMIT to check your answers. Click NEXT to get started.


