
Bohr and The Hydrogen Atom
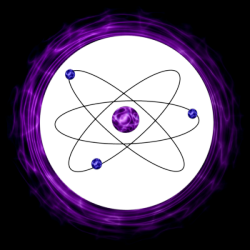 Niels Bohr developed one of the most recognizable atomic models. Building on Rutherford's model, Bohr's model placed the electrons outside of the nucleus on specific energy levels. It is due to this energy that the electrons do not fall into nucleus. The further the electron is from the nucleus, the higher the energy level. Bohr also established that electrons cannot reside between these specific energy levels. His work with the hydrogen spectrum led to the analysis of various types of atoms.
Niels Bohr developed one of the most recognizable atomic models. Building on Rutherford's model, Bohr's model placed the electrons outside of the nucleus on specific energy levels. It is due to this energy that the electrons do not fall into nucleus. The further the electron is from the nucleus, the higher the energy level. Bohr also established that electrons cannot reside between these specific energy levels. His work with the hydrogen spectrum led to the analysis of various types of atoms.




