
Moseley and Periodic Law
In 1869, Dmitri Mendeleev published his now famous version of the periodic table. He arranged the table so that groups of elements with similar properties were together. In most instances, this meant that the elements were placed in order of increasing mass. But a few were not. Why? This question was not answered until more than forty years later by Henry Moseley.
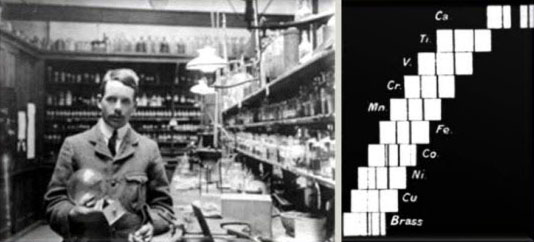
Henry Moseley (left), and a photograph of the X-ray spectra of different anodes from his experiment (right)
In 1911, Ernest Rutherford discovered the proton. Shortly after Rutherford’s Gold Foil Experiment, an intelligent graduate assistant of Rutherford’s, Henry Moseley, derived the relationship between X-ray frequency and number of protons. Moseley was only twenty-five years old when he used this information to rearrange the elements according to increasing atomic numbers, and not atomic masses as Mendeleev had done. Today, the periodic table is arranged according to atomic number, or number of protons. Moseley’s arrangement seemed to clear up the inconsistencies with Mendeleev’s table. The Periodic Law, as stated by Mendeleev, said that properties of the elements occur in a periodic fashion when elements are arranged in order of increasing atomic masses. Moseley restated the Periodic Law to say that instead of the properties of elements being a periodic function of their masses, the properties are a periodic function of the atomic number. Today, this law is known both as Periodic Law and Mendeleev’s Law.
The Periodic Law
The Periodic Law states that many of the physical and chemical properties of the elements tend to recur in a systematic manner with increasing atomic number. Progressing from the lightest to the heaviest atoms, certain properties of the elements approximate those of precursors at regular intervals; two, eight, eighteen, and thirty-two.
For example, the 2d element helium is similar in chemical behavior to the neon (atomic number 10), as well as to the argon (atomic number 18), krypton (atomic number 36), xenon (atomic number 54), and radon (atomic number 86). Using the Periodic Law, elements can be linked together by their chemical properties.

Glenn Seaborg in his lab
Unfortunately, Henry Moseley was tragically killed in World War I only two years later. Many scientists believe Moseley would have been awarded the Noble Prize in Physics in 1916, if it was not for his death. Because of this tragedy, the British government no longer assigns prominent scientists to combat duty.
The last major change to the periodic table was when Glenn Seaborg discovered a group of elements called the transuranium elements and reconfigured the periodic table by placing the lanthanide and actinide series at the bottom of the table. This was considered very radical at the time. Eventually, his table was accepted and he was awarded the Nobel Prize in Chemistry in 1951. He also has an element named after him. This element has the atomic number of 106, and it is known as seaborgium (Sg).
Moseley and Periodic Law Review

![]() As you can tell, the arrangement of the periodic table was decided by the dedicated laboratory work of a few scientists. Now that you have learned about Henry Moseley, Periodic Law, and Glenn Seaborg, it is time to check your knowledge in this non-graded activity. Read each statement and decide if it is True or False. Then, click SUBMIT to check your response. Click the interactivity thumbnails, and then click NEXT to get started.
As you can tell, the arrangement of the periodic table was decided by the dedicated laboratory work of a few scientists. Now that you have learned about Henry Moseley, Periodic Law, and Glenn Seaborg, it is time to check your knowledge in this non-graded activity. Read each statement and decide if it is True or False. Then, click SUBMIT to check your response. Click the interactivity thumbnails, and then click NEXT to get started.


