
Ionic Formula Writing and Naming: Binary
Metals, Nonmetals, or Semi-Metals
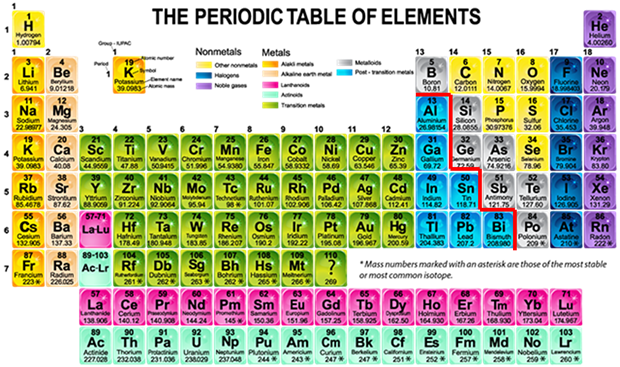
![]() At this point in your study of chemistry, you are probably very familiar with the structure of the periodic table. Take a few moments to look at the key for the periodic table shown in the image on this page. Different colors are used to separate metals and nonmetals. You can see from the periodic table, most elements are metals. Metals are used in thousands of applications like car batteries, rocket engines, smoke detectors, fireworks, and coins just to name a few. Why is it important that you review this information? Because it is important when you name formulas. Remember, metals and nonmetals combine to form an ionic bond. A red line has been drawn on this periodic table. This line separates the metalloids from transition metals. This line represents an area on the periodic table where metals are considered semi-metals, or poor metals.
At this point in your study of chemistry, you are probably very familiar with the structure of the periodic table. Take a few moments to look at the key for the periodic table shown in the image on this page. Different colors are used to separate metals and nonmetals. You can see from the periodic table, most elements are metals. Metals are used in thousands of applications like car batteries, rocket engines, smoke detectors, fireworks, and coins just to name a few. Why is it important that you review this information? Because it is important when you name formulas. Remember, metals and nonmetals combine to form an ionic bond. A red line has been drawn on this periodic table. This line separates the metalloids from transition metals. This line represents an area on the periodic table where metals are considered semi-metals, or poor metals.

