
VSEPR Theory and Orbital Hybridization
 Analyzing Lewis dot structures to try to determine molecular shape is not an easy task. Chemists need the information on a compound's shape to understand and predict the chemical properties of the compound. VSEPR, or Valence Shell Electron Pair Repulsion, theory is a model that allows chemists to determine the molecular shapes of compounds with ease. View this presentation to learn more about the VSEPR model.
Analyzing Lewis dot structures to try to determine molecular shape is not an easy task. Chemists need the information on a compound's shape to understand and predict the chemical properties of the compound. VSEPR, or Valence Shell Electron Pair Repulsion, theory is a model that allows chemists to determine the molecular shapes of compounds with ease. View this presentation to learn more about the VSEPR model.
View a printable version of this interactivity.
Orbital Hybridization
Chemist Linus Pauling developed a theory of hybridization to explain the structure of compounds like methane (CH4). If chemists relied solely on the Valence Bond Theory, which states that bonding only occurs between two atoms sharing electrons, then carbon could only create two covalent bonds (CH2). You know that this is not true. Hybridization occurs when atomic orbitals mix to form new orbitals. The new orbitals have the same electron capacity as all of the orbitals combined. The property of the new orbital is an average of the combining orbitals.
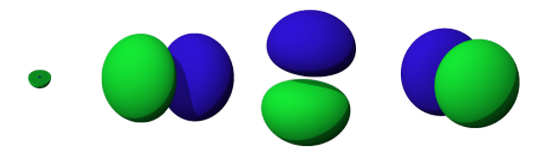
The shapes of the 2s, 2px, 2py, and 2pz orbitals
Carbon’s electron configuration is 1s22s22p2. The four electrons from 2s and 2p are available to form chemical bonds. Do you remember what the electron orbital shapes look like? Please take a moment to view the orbitals in the image above.
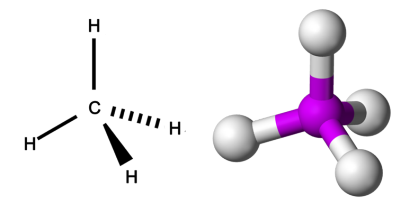
The tetrahedral shape of methane's hybrid orbital
In carbon, the 2s orbital and the 2p orbital hybridize to form a hybrid orbital called sp3. The sp3 orbital can hold eight electrons. The sp3 orbital will have a tetrahedral shape, as shown in the image above. There are plenty of other elements that go through the process of hybridization.

![]() Now that you have investigated molecular shape and VSEPR theory, review your knowledge in this non-graded activity. Read each question and select the appropriate answer. Then, click SUBMIT to check your response. Click on the interactivity thumbnail, and then click NEXT to get started.
Now that you have investigated molecular shape and VSEPR theory, review your knowledge in this non-graded activity. Read each question and select the appropriate answer. Then, click SUBMIT to check your response. Click on the interactivity thumbnail, and then click NEXT to get started.


