
Mass to Mass Stoichiometry
 Now that you practiced dimensional analysis calculations with something delicious like chocolate chip cookies, doing mass to mass stoichiometry problems is a breeze. The idea is exactly the same. In the cookie “reaction”, you saw that the ingredients did not react on a gram to gram basis. It was necessary to look at the coefficients of the overall reaction and then convert each of those to grams. You will also have to remember calculations involving mole ratios. In order to compare the mass of one substance in a reaction to the mass of another substance in the reaction, it is necessary to convert both to moles and then use the mole ratio. To learn how to complete mass to mass stoichiometry problems view the example problem below.
Now that you practiced dimensional analysis calculations with something delicious like chocolate chip cookies, doing mass to mass stoichiometry problems is a breeze. The idea is exactly the same. In the cookie “reaction”, you saw that the ingredients did not react on a gram to gram basis. It was necessary to look at the coefficients of the overall reaction and then convert each of those to grams. You will also have to remember calculations involving mole ratios. In order to compare the mass of one substance in a reaction to the mass of another substance in the reaction, it is necessary to convert both to moles and then use the mole ratio. To learn how to complete mass to mass stoichiometry problems view the example problem below.
If there are 21.3 grams of nitrogen, how many grams of ammonia can be produced (assuming that there is enough hydrogen)?
Please note: The coefficient of 1 in front of N2 does not have to be written; it was just written here to coincide with the equation written out in words.
- First convert grams of nitrogen to moles of nitrogen.
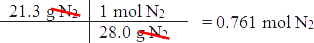
- Then, determine how many moles of ammonia will react with _____ moles of nitrogen.

- Next, convert moles of ammonia to grams of ammonia.
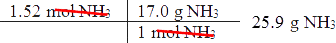
Of course, you can solve this entire problem in one step using dimensional analysis.
Mass to Mass Stoichiometry Review

![]() Now that you have learned how to make calculations using mass to mass stoichiometry, review your knowledge in this non-graded activity. Use your cursor to select the conversion factor that will be used in each dimensional analysis. Then, click SUBMIT to check your response. Click on the interactivity thumbnail, and then click NEXT to get started.
Now that you have learned how to make calculations using mass to mass stoichiometry, review your knowledge in this non-graded activity. Use your cursor to select the conversion factor that will be used in each dimensional analysis. Then, click SUBMIT to check your response. Click on the interactivity thumbnail, and then click NEXT to get started.


