
Heat Changes Associated with Chemical Reactions, Hess’ Law, and Reaction Profiles
Systems and Surroundings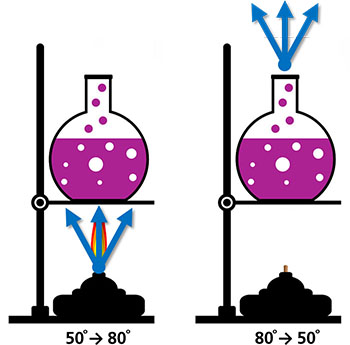
In thermochemistry, the terms system and surroundings have precise and important scientific meanings. The system is the object, or collection of objects, that you are studying. The surroundings include everything outside of the system that exchanges energy with the system. For example, in the picture, the liquid is considered the system and anything that is in thermal contact with the water would be considered the surroundings. The hot plate, the air above and the glass of the beaker are all considered to be surroundings.
Many reactions occur in water. This is called reacting in aqueous conditions. In these reactions, water is part of the surroundings, not the system. The chemicals dissolved in the water are located in the test tube and react with the chemicals that are dissolved in the water in the beaker. The chemicals are the system. The test tube, the beaker, the water, the air around the containers, the desktop and the chemist’s hands are all surroundings.
Types of Thermochemical Processes
 When studying the transfer of heat, there are some principles that are important to learn prior to learning about the different types of thermochemical processes. First, heat always transfers from an object of higher temperature to an object of lower temperature. Second, the transfer of energy continues to occur until both objects are at the same temperature. In thermochemistry, there are two different types of heat transfer processes. In this activity, click on each of the questions to learn about the two types of thermochemical processes.
When studying the transfer of heat, there are some principles that are important to learn prior to learning about the different types of thermochemical processes. First, heat always transfers from an object of higher temperature to an object of lower temperature. Second, the transfer of energy continues to occur until both objects are at the same temperature. In thermochemistry, there are two different types of heat transfer processes. In this activity, click on each of the questions to learn about the two types of thermochemical processes.
View a printable version of the interactivity.
Enthalpy and Hess's Law
 When discussing the energy changes associated with chemical reactions, the heat content is usually referred to as the enthalpy. Enthalpy is a measure of the total energy of a thermochemical system. The unit of measurement for enthalpy in the International System of Units (SI) is the joule. However, the calorie is still used as a unit of measurement by some chemists. View this presentation to learn about enthalpy and Hess’s Law.
When discussing the energy changes associated with chemical reactions, the heat content is usually referred to as the enthalpy. Enthalpy is a measure of the total energy of a thermochemical system. The unit of measurement for enthalpy in the International System of Units (SI) is the joule. However, the calorie is still used as a unit of measurement by some chemists. View this presentation to learn about enthalpy and Hess’s Law.
View a printable version of the interactivity or a printable version of the Standard Enthalpy of Formation for Various Compounds Table.
Thermochemical Processes, Enthalpy, and Hess's Law Review
![]()
 Now that you have explored thermochemical processes, enthalpy, and Hess's Law, complete this activity to test your knowledge. In this non-graded activity, answer all of the questions by following the directions on each question slide and click SUBMIT to check your response(s). Click on the interactivity thumbnail, and then click NEXT to get started.
Now that you have explored thermochemical processes, enthalpy, and Hess's Law, complete this activity to test your knowledge. In this non-graded activity, answer all of the questions by following the directions on each question slide and click SUBMIT to check your response(s). Click on the interactivity thumbnail, and then click NEXT to get started.


