
Catalysis and Activation Energy
 Using a catalyst to accelerate a chemical reaction has been observed since the early nineteenth century. These accelerators were used in soap making and wine making. Catalysts are also used to help bread dough rise. Catalysts were used in the everyday home without a chemist even being able to describe their purpose. Can you think of any catalysts you have observed?
Using a catalyst to accelerate a chemical reaction has been observed since the early nineteenth century. These accelerators were used in soap making and wine making. Catalysts are also used to help bread dough rise. Catalysts were used in the everyday home without a chemist even being able to describe their purpose. Can you think of any catalysts you have observed?
As chemists continued the study of these reaction accelerators, they discovered that catalysts only accelerate the chemical reaction. This happens because the catalyst provides a way for the reaction to occur with less activation energy. If the activation energy in a reaction is high, the reaction will most likely stop. If the activation energy of the reaction is low, the reaction will occur rapidly. It is important to note that a catalyst is neither a reactant nor product. The reaction usually occurs in steps and can form an intermediate, which cannot be formed without the catalyst.
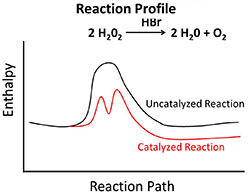 The image shown is a reaction profile. In the reaction, hydrogen peroxide produces water and oxygen. This decomposition reaction occurs slowly at room temperature. However, if the catalyst hydrobromic acid is added, the reaction occurs quicker. Notice from the reaction profile that the reaction has two intermediate steps.
The image shown is a reaction profile. In the reaction, hydrogen peroxide produces water and oxygen. This decomposition reaction occurs slowly at room temperature. However, if the catalyst hydrobromic acid is added, the reaction occurs quicker. Notice from the reaction profile that the reaction has two intermediate steps.
Catalysts are classified into two different categories. In a homogeneous catalyst, the reactants and catalyst are in the same phase. Generally these reactions are simpler and easy to understand than heterogeneous catalyst reactions. In a heterogeneous catalyst, the catalyst is in a different phase from the reactants and products. Usually, the catalyst is a solid and the reactants and products are gases or liquids. The reaction occurs on the solid surface of the catalyst. This type of reaction is commonly seen in a battery cell.
Why study how a catalyst affects a reaction? Catalysts are very important processes as they provide great aid in producing most of the manufactured chemicals. Chemical engineers from all types of different fields use catalysts, study the reactions, and manufacture chemicals. One of the most common substances comes from a catalyst reaction. In 1908, chemist Fritz Haber used iron as a catalyst to combine nitrogen and hydrogen producing ammonia. Worldwide ammonia is a commonly used chemical.
Review Facts about Catalysts
 As you just learned, enzymes are important in the development of new chemical compounds. Before you learn about a specific type of catalyst called an enzyme, review what you already know in this interactivity. In this activity, click the checkboxes from the clipboard to review facts about catalysts.
As you just learned, enzymes are important in the development of new chemical compounds. Before you learn about a specific type of catalyst called an enzyme, review what you already know in this interactivity. In this activity, click the checkboxes from the clipboard to review facts about catalysts.
View a printable version of the interactivity.
Enzymes
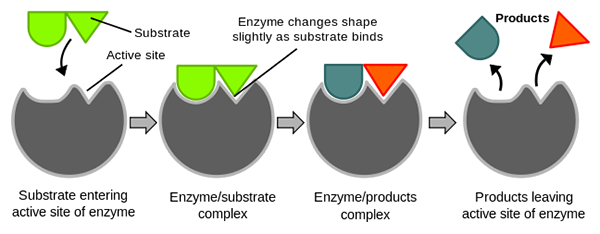
Diagram showing how enzymes connect with substrates creating a product
In the field of biochemistry specific catalysts exist called enzymes. Enzymes are the biological substances that act as catalysts and help complex reactions occur everywhere in life. Enzymes are known to take on a specific role, only working with specific molecules.
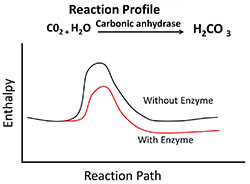 Enzymes work by completing steps in order. You can view each of these steps in the image on this page. In order for enzymes to work, the enzyme and substrate must be located in the same area. The substrate is the biological molecule that the enzyme works on. The enzyme connects with the substrate at a location called the active site. This binding lowers the activation energy of the reaction. Next, a process of catalysis takes place transforming the substrate into a new substance. Lastly, the enzyme releases the new substance and finds another substrate. The new substance is the product. Take a moment to view the profile when carbon dioxide and water react forming carbonic acid. This reaction takes place slowly. However, if a certain enzyme called carbonic anhydrase is added to the reaction, the reaction accelerates.
Enzymes work by completing steps in order. You can view each of these steps in the image on this page. In order for enzymes to work, the enzyme and substrate must be located in the same area. The substrate is the biological molecule that the enzyme works on. The enzyme connects with the substrate at a location called the active site. This binding lowers the activation energy of the reaction. Next, a process of catalysis takes place transforming the substrate into a new substance. Lastly, the enzyme releases the new substance and finds another substrate. The new substance is the product. Take a moment to view the profile when carbon dioxide and water react forming carbonic acid. This reaction takes place slowly. However, if a certain enzyme called carbonic anhydrase is added to the reaction, the reaction accelerates.
Catalysis and Activation Energy Review
![]()
 Now that you have learned about catalysis and activation energy, it is time to check your knowledge. In this non-graded activity, read each statement and decide if it is True or False. To check your responses, click SUBMIT. Click on the interactivity thumbnail, and then click NEXT to get started.
Now that you have learned about catalysis and activation energy, it is time to check your knowledge. In this non-graded activity, read each statement and decide if it is True or False. To check your responses, click SUBMIT. Click on the interactivity thumbnail, and then click NEXT to get started.


