
Phase Diagrams and Vapor Pressure Diagrams
Phase Diagrams
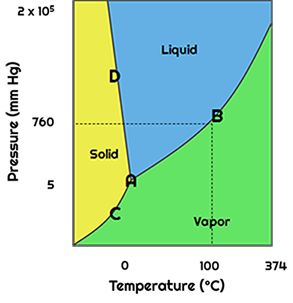 Phase diagrams are very simple and show the boundary between each phase. This boundary is observed as a line. If you were to cross the line from one phase to the next, a phase change occurs. In order to interpret this diagram, you must remember the different phase changes. For example, when a liquid changes to a gas, vaporization occurs. If a vapor changes to a liquid, condensation occurs. If a solid changes into a liquid, melting occurs. If a liquid freezes, it will become solid. Lastly, the process that changes directly from solid to vapor is sublimation. Deposition is the opposite of this process. All of these phase changes, along with the temperature and pressure associated with each change, are observed on the phase diagram.
Phase diagrams are very simple and show the boundary between each phase. This boundary is observed as a line. If you were to cross the line from one phase to the next, a phase change occurs. In order to interpret this diagram, you must remember the different phase changes. For example, when a liquid changes to a gas, vaporization occurs. If a vapor changes to a liquid, condensation occurs. If a solid changes into a liquid, melting occurs. If a liquid freezes, it will become solid. Lastly, the process that changes directly from solid to vapor is sublimation. Deposition is the opposite of this process. All of these phase changes, along with the temperature and pressure associated with each change, are observed on the phase diagram.
Traditionally, you might think boiling occurs when you heat a liquid to 100°C. The phase diagram shows that this is not always the case. Normal boiling point is the temperature that a substance boils under at standard pressure. Remember, the standard pressure conditions are 1.0 atmospheres, 101.325 kPa, or 760.0 mmHg. What happens when this pressure is not standard? The vapor pressure diagram shows you that boiling, as well as other phase changes, can occur at different temperatures. Notice that the boiling temperature changes along this line as the pressure changes.
Vapor Pressure Diagrams
 Vapor pressure diagrams provide scientists with a lot of information, specifically the point at which temperature and pressure allow a substance to vaporize. These diagrams can display information about several substances all on the same graph. View this presentation to learn more about vapor pressure and vapor pressure diagrams.
Vapor pressure diagrams provide scientists with a lot of information, specifically the point at which temperature and pressure allow a substance to vaporize. These diagrams can display information about several substances all on the same graph. View this presentation to learn more about vapor pressure and vapor pressure diagrams.
View a printable version of the interactivity.
Phase Diagrams and Vapor Pressure Diagrams Review

![]() Now that you have explored both phase and vapor pressure diagrams, review your knowledge in this non-graded activity. In this activity, answer all of the questions by following the instructions on each question slide. Click SUBMIT to check your response(s). Click on the interactivity thumbnail, and then click NEXT to get started.
Now that you have explored both phase and vapor pressure diagrams, review your knowledge in this non-graded activity. In this activity, answer all of the questions by following the instructions on each question slide. Click SUBMIT to check your response(s). Click on the interactivity thumbnail, and then click NEXT to get started.


