
Matter
Everything that can be observed is made of matter. Matter can have infinite properties and forms, but it always has mass and volume. All matter is made of tiny particles called atoms. Matter can exist in four different phases: solid, liquid, gas, and plasma. Hover your cursor over each of the states of matter shown below to learn more about each.
View a printable version of the interactivity.

![]() Have you ever watched a pot of water boil? When water is exposed to enough heat, it will begin to bubble and boil. Some of the water will escape the pot as steam. Droplets of steam condense back into water droplets on the sides and top of the pot. You are watching water go through a phase change. Energy can cause matter to change forms. Water changes forms without changing its chemical composition. View the video clip States of Matter | Science Trek, from eMediaVA℠ to learn more about the properties of matter and to view examples of matter changing phases.
Have you ever watched a pot of water boil? When water is exposed to enough heat, it will begin to bubble and boil. Some of the water will escape the pot as steam. Droplets of steam condense back into water droplets on the sides and top of the pot. You are watching water go through a phase change. Energy can cause matter to change forms. Water changes forms without changing its chemical composition. View the video clip States of Matter | Science Trek, from eMediaVA℠ to learn more about the properties of matter and to view examples of matter changing phases.
Atomic Structure
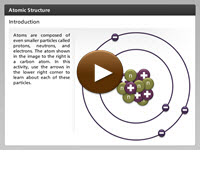 Knowing some basic chemistry is an important part of being an Earth scientist. Everything in the natural world is composed of matter. Matter makes atoms, which are the building blocks for elements. Elements combine to form complex compounds. This is how the Earth only has 118 elements, but well over 3000 known minerals. Atoms are composed of even smaller particles called protons, neutrons, and electrons. Each of these particles plays an important role in atomic structure. In this activity, you will learn about each of these particles. Click the player button to get started.
Knowing some basic chemistry is an important part of being an Earth scientist. Everything in the natural world is composed of matter. Matter makes atoms, which are the building blocks for elements. Elements combine to form complex compounds. This is how the Earth only has 118 elements, but well over 3000 known minerals. Atoms are composed of even smaller particles called protons, neutrons, and electrons. Each of these particles plays an important role in atomic structure. In this activity, you will learn about each of these particles. Click the player button to get started.
View a printable version of the interactivity.
The Periodic Table of Elements
 The periodic table holds a lot of information that is important to Earth scientists. By providing a standardized record of all of the known elements, Earth scientists can easily research what elements compose minerals, rocks, the atmosphere, and the ocean water. View this presentation to learn about the periodic table and the information it provides. Click the player button to begin.
The periodic table holds a lot of information that is important to Earth scientists. By providing a standardized record of all of the known elements, Earth scientists can easily research what elements compose minerals, rocks, the atmosphere, and the ocean water. View this presentation to learn about the periodic table and the information it provides. Click the player button to begin.
View a printable version of the interactivity.
Isotopes
 Certain atoms of the same element have different numbers of protons and neutrons. This causes the element to become unstable. Earth scientists can use some isotopes in order to find the age of certain objects. But, what is an isotope exactly? View this presentation to learn about isotopes and observe examples of carbon isotopes. Click the player button to get started.
Certain atoms of the same element have different numbers of protons and neutrons. This causes the element to become unstable. Earth scientists can use some isotopes in order to find the age of certain objects. But, what is an isotope exactly? View this presentation to learn about isotopes and observe examples of carbon isotopes. Click the player button to get started.
View a printable version of the interactivity.
Chemical Bonding
 In nature, most matter exists as a result of combinations of two or more elements. These chemical combinations are termed compounds. A compound is a substance made of atoms from more than one element. Compounds are held together by a chemical bond and can be chemically broken down into separate elements. Did you know your kitchen is full of compounds? Water is a compound, as well as salt. Water forms when two hydrogen atoms combine with one oxygen atom (H2O). Salt forms when one sodium atom combines with one chlorine atom (NaCl).
In nature, most matter exists as a result of combinations of two or more elements. These chemical combinations are termed compounds. A compound is a substance made of atoms from more than one element. Compounds are held together by a chemical bond and can be chemically broken down into separate elements. Did you know your kitchen is full of compounds? Water is a compound, as well as salt. Water forms when two hydrogen atoms combine with one oxygen atom (H2O). Salt forms when one sodium atom combines with one chlorine atom (NaCl).
Three types of chemical bonds occur to form chemical compounds. Covalent bonding takes place when atoms share electrons. One of the most common minerals on Earth is a result of covalent bonding. The mineral quartz forms as a result of the electrons shared by one silicon atom and two oxygen atoms. Ionic bonds form between positive and negative ions. Salt is a result of ionic bonding. Both sodium and chlorine in their natural forms are lethal to humans. Sodium reacts with your skin if it is held in your hand. Sodium also reacts with water. Chlorine is most commonly known as a green poisonous gas. When they combine, they form salt, a substance used to season food. Metallic bonds form when the electrons are shared by metal ions. The sharing of electrons allows metals to have properties like conductivity, being ductile, and malleable. Metals also have the ability to be drawn into wires.
Matter Review
![]()
 Now that you have explored the nature of matter and some basic chemistry, review your knowledge. In the non-graded activity, answer all of the questions by following the directions associated with each question. Click the player button to get started.
Now that you have explored the nature of matter and some basic chemistry, review your knowledge. In the non-graded activity, answer all of the questions by following the directions associated with each question. Click the player button to get started.






