
Salinity
 Dissolved Substances
Dissolved Substances
Scientific Investigation
![]() Before you begin the scientific investigation below, make sure to download the Dissolved Substances Scientific Investigation Report. As you complete this scientific investigation, fill in any needed information on the report template. If you need more information about each section of the report, please visit the Developmental Module.
Before you begin the scientific investigation below, make sure to download the Dissolved Substances Scientific Investigation Report. As you complete this scientific investigation, fill in any needed information on the report template. If you need more information about each section of the report, please visit the Developmental Module.
This scientific investigation is available below or in a printable version.
Introduction
Seawater owes its salty taste to the high quantities of dissolved salts, gases, and other minerals in the water. Scientists measure the amount of dissolved substances in seawater to determine its salinity. Although the salinity of seawater varies from place to place, in general it has a salinity of about 3.5%. When water cycles into seawater through precipitation, runoff, and melting icebergs, the concentration of dissolved substances decreases, thus lowering the overall salinity. When water cycles out of the seawater through the formation of sea ice and evaporation, the concentration of dissolved substances increases, thus raising the overall salinity.
Objectives
In this scientific investigation, you will:
- explore the relationships between salinity, dissolved substances, and the quantity of water.
- observe the changes in concentration when a solvent is added to a solute.
Hypothesis
Using the Procedure and Data Collection section below, read through the procedural information for this scientific investigation. Based on your understanding of the procedure, develop your own hypotheses which describe your expected results. What do you think the relationship between the solute (water), the solvent (salt), and the solution (the mixture) will be when different amounts of each are added and subtracted? Record these hypotheses in the Hypothesis section of your Dissolved Substances Scientific Investigation Report.
Required Simulation
Sugar and Salt Solutions Simulation
(click on image below to run simulation)
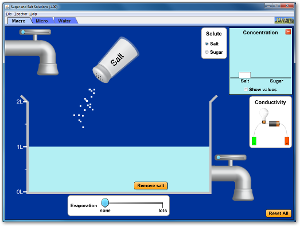 |
Provided by:
PhET Interactive Simulations
University of Colorado
http://phet.colorado.edu
Procedure and Data Collection
 Simulation Set-Up (see image at right)
Simulation Set-Up (see image at right)
- Open the Sugar and Salt Solutions simulation.
- In the upper left corner of the simulation, make sure that the Macro tab is selected.
- In the Solute box, click the Salt button.
- In the Concentration box, click the check box to Show values.
Effect of Added Solvent on the Concentration of the Solution
- Use your cursor to grab and shake the salt shaker, shaking it eight times to add salt to the water.
- Remove your cursor from the salt shaker.
- In the Data section of your Dissolved Substances Scientific Investigation Report, record the effect of added solvent (salt) on the salinity of the solution. How many mol/L of salt is present in the solution?
Effect of Added Solute on the Concentration of the Solution
- Use your cursor to move the button on the upper left water faucet to the right, adding 0.5 L of water into the solution.
- Turn the water off.
- In the Data section of your Dissolved Substances Scientific Investigation Report, record the effect of added solute (water) on the salinity of the solution. How many mol/L of salt is present in the solution?
Effect of Solution Quantity on the Concentration of the Solution
- Use your cursor to move the button on the lower right water faucet to the right, removing 1 L of the solution.
- Turn the water off.
- In the Data section of your Dissolved Substances Scientific Investigation Report, record the effect of removed solution on the salinity of the solution. How many mol/L of salt is present in the solution?
Data
Use the data table provided on your Dissolved Substances Scientific Investigation Report to record your data from this scientific investigation. The data table is also shown below:
Effect of Added Solvent and Solute on Salinity
| Change | Amount Added | Salinity/Salt Concentration (mol/L) |
| Added solvent (salt) | ||
| Added solute (water) | ||
| Removed solution |
Data Analysis
In the Data Analysis section of your Dissolved Substances Scientific Investigation Report, provide responses to the following questions:
- Describe what happened to the salinity of the solution when you added salt. Explain why.
- Describe what happened to the salinity of the solution when you added water. Explain why.
- Describe what happened to the salinity of the solution when you removed solution. Explain why.
- List all of the different ways that you can change the concentration of a solution.
Conclusion
Using the Conclusion section of your Dissolved Substances Scientific Investigation Report, compose three to four sentences describing an overall conclusion about the relationship between the solute (water), the solvent (salt), and the solution (the mixture) will be when different amounts of each are added and subtracted, based on your data. Were your hypotheses true or false, and how do you know? Use the data and notes that you collected from your simulation experience to form your conclusion. Make sure that you include information that you gained from data analysis to support your conclusion.
Experimental Sources of Error
On your Dissolved Substances Scientific Investigation Report, provide responses to the following questions: Are there any sources of error? If so, what are they, and what could be done to minimize error?
![]() Once you have completed the Dissolved Substances Scientific Investigation Report, please submit your work to the dropbox.
Once you have completed the Dissolved Substances Scientific Investigation Report, please submit your work to the dropbox.



