
Pascal’s Principle
Pressure exerted by water is controlled by its density and depth. Now, explore Pascal’s Principle, how it affects the pressure under water, and how it is used to design hydraulics systems.
![]()
 The man in the photo on the right is Jacques Cousteau. Jacques Cousteau made the first underwater film in 1943. He is famous for his underwater explorations that he shared with the world through film.
The man in the photo on the right is Jacques Cousteau. Jacques Cousteau made the first underwater film in 1943. He is famous for his underwater explorations that he shared with the world through film.

The picture at left shows typical scuba gear. Each piece of gear has a specific purpose. When you plan your diving trip you will consider how physics affects diving.
When pressure is applied to a fluid that is at rest, the pressure is transmitted equally throughout the fluid.
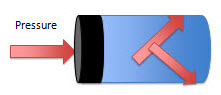 The pressure exerted by the piston pushes on the fluid in this container. That pressure is transmitted all the way through the container.
The pressure exerted by the piston pushes on the fluid in this container. That pressure is transmitted all the way through the container.
Hydraulics takes advantage of this basic principle in physics.
Pressure Under Water
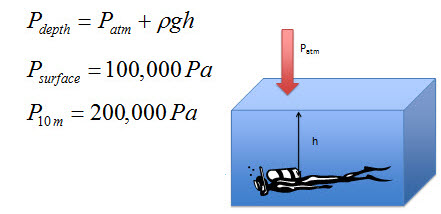 When the diver is at the surface, the pressure on her is one hundred thousand pascals.
When the diver is at the surface, the pressure on her is one hundred thousand pascals.
When the diver is ten meters below the surface, the pressure on her is two hundred thousand pascals. This is twice as much pressure compared to the surface. How does the increased pressure affect the diver?
Pressure changes with depth in a static fluid; however, the pressure of the atmosphere also affects the pressure at the bottom of the container. For example, if you are diving in a lake, not only does the water above you exert pressure on you, the pressure of the atmosphere, which pushes down on the surface of the lake, is transmitted through the water to you.
You can figure out the actual pressure under the water by adding the pressure exerted by the water to the pressure the atmosphere exerts on the surface of the water.
You might remember that in a previous topic we figured out a rule of thumb for how much water depth it takes to equal the pressure of the atmosphere. The rule was that ten point two meters of fresh water exerts as much pressure as the atmosphere. If you go diving, every time you go another ten meters in depth, the pressure increases by one hundred thousand pascals.
When the diver went from the surface to ten meters below the surface, the pressure doubled. How does this affect her?
The solubility of a gas in a liquid is dependent upon pressure. As pressure increases, more gas is able to dissolve. When a diver descends, the increased pressure allows more nitrogen from the air she is breathing to dissolve in her blood. When she ascends, if she goes too fast, nitrogen bubbles can form in her blood. This is like when you open a bottle of soda. Lowering the pressure causes carbon dioxide bubbles to come out of solution. If the bubbles are too large, they can block circulation and causes other serious health problems such as weakness, paralysis, nausea and worse. Maybe you have heard of “the bends." This is caused by nitrogen bubbles in the blood causing pain in the small veins of joints. Divers bend these joints to relieve this pain. This is why they call it “the bends." You can see that pressure changes are very important for divers to pay attention to. Divers follow time tables to control the rate that they ascend to the surface for this reason.
Why do divers need pressurized air to breathe underwater?
How deep could you dive with a snorkel (breathing tube)?
These questions are important to our diving trip. The only way the diver can breathe is if his or her lung muscles can work against the pressure difference inside and outside the body. The longest snorkel length is 40 cm.
So how does this relate to needing pressurized air? The pressurized air raises the pressure inside the lung, making it closer to the pressure outside the body, so the lung muscles don’t have to work as hard. You may wonder how much pressure is in the air in the tank. A device called a regulator keeps the pressure of the air about one hundred and fifty psi above the pressure surrounding the diver. The regulator adjusts itself for pressure changes that occur with the diver’s depth changes. This way the pressure of the air that enters the diver’s lungs is kept at safe levels.
Pascal's Principle
 A common application of this is a hydraulic lift used to raise a car off the ground, so it can be repaired at a garage. A small force applied to a small-area piston is transformed to a large force at a large-area piston. If a car sits on top of the large piston, it can be lifted by applying a relatively small force to the smaller piston, the ratio of the forces being equal to the ratio of the areas of the pistons.
A common application of this is a hydraulic lift used to raise a car off the ground, so it can be repaired at a garage. A small force applied to a small-area piston is transformed to a large force at a large-area piston. If a car sits on top of the large piston, it can be lifted by applying a relatively small force to the smaller piston, the ratio of the forces being equal to the ratio of the areas of the pistons.
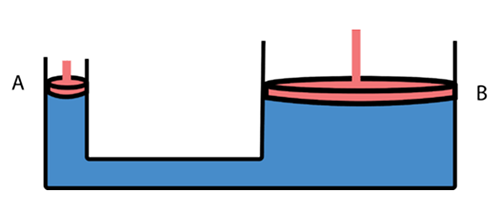
How can a single person lift the end of an automobile with one hand? The hydraulic jack shown below uses a compressed liquid to develop a mechanical advantage according to Pascal’s Principle.
Pascal's Principle: Pressure applied to an enclosed fluid is transmitted undiminished to every part of the fluid, as well as to the walls of the container.
The sketch below is an example of a hydraulic lift. We have a confined liquid in contact with two pistons (A and B) of different sizes. The pressure of the liquid on these two pistons is the same (Pascal's principle). Therefore, FA=FB ,
and FA / AA= FB /AB and FA / FB = AA / AB
Suppose that the area of piston A is 4.0 cm2 and the area of piston B is 200. cm2. If we place an automobile weighing 10,000 N on piston B, we can lift that car by exerting a force of 200 N on piston A. This is another form of simple machine and its ideal mechanical advantage is 50. The ideal mechanical advantage of a hydraulic lift equals the ratio of the large piston area to the small piston area.
Pascal's Principle Practice
![]()
 In the practice problems in this interactivity, you will apply what you have learned about Pascal's Principle and hydraulics. Please be sure to try each problem before checking your answers. Click the player to get started.
In the practice problems in this interactivity, you will apply what you have learned about Pascal's Principle and hydraulics. Please be sure to try each problem before checking your answers. Click the player to get started.


