
Atoms - The Basics
Build an Atom Scientific Investigation

![]() Before you begin the scientific investigation below, make sure to download the Build an Atom Scientific Investigation Report. As you complete this scientific investigation, fill in any needed information on the report template. If you need more information about each section of the report, please visit the Developmental Module.
Before you begin the scientific investigation below, make sure to download the Build an Atom Scientific Investigation Report. As you complete this scientific investigation, fill in any needed information on the report template. If you need more information about each section of the report, please visit the Developmental Module.
This scientific investigation is available below or in a printable version.
Introduction
Atoms are made of protons, electrons, and maybe neutrons, depending on the element. Adding or subtracting these components can alter the element, the charge, and the mass. As you have learned, the atom is the basic building block of all matter. Matter is made of elements, and the periodic table organizes these elements. Hydrogen is the first element on the periodic table.
Objectives
In this scientific investigation, you will:
- explore the relationship among protons, electrons, and neutrons;
- explore how the addition or subtraction of protons, electrons, and neutrons changes the element, charge, and mass; and
- explore how to use the element name, mass, and charge to determine the number of protons, neutrons, and electrons in an atom of a particular element.
Hypothesis
Using the Procedure and Data Collection section below, read through the procedural information for this scientific investigation. Based on your understanding of the procedure, develop your own hypotheses, which describe your expected results. Specifically, what do you think the relationship among protons, electrons, and neutrons will be, especially if any of the components are added or subtracted from the atom? Record these hypotheses on your Build an Atom Scientific Investigation Report in the Hypothesis section.
Required Simulation
Build and Atom Simulation
(click on image below to access simulation)
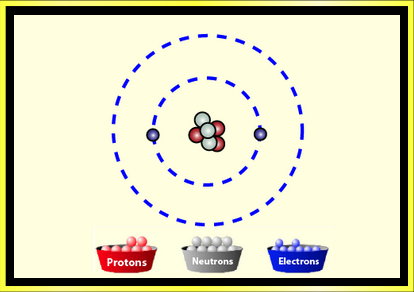
Provided by:
PhET Interactive Simulations
University of Colorado
http://phet.colorado.edu
Procedure and Data Collection
Simulation Set-Up
- On the Build an Atom webpage, click on the option for Atom.
- Under "Model," make sure the simulation is in the Orbits mode.
- Under "Show," make sure that Element Name, Neutral/Ion, and Stable/Unstable are all checked.
- Click on any of the
 symbols so that you will be able to see the changes in the Element, the Net Charge, or the Mass Number.
symbols so that you will be able to see the changes in the Element, the Net Charge, or the Mass Number.
Build a Hydrogen Atom
- Find Hydrogen (H) in the box labeled Elements.
- From the "baskets" provided on the bottom of the screen, use your cursor to drop one proton (red) and one neutron (silver) in the middle of the atom model. Then, use your cursor to drop one electron (blue) onto the atom. Complete the first row of the Build a Hydrogen Atom data table in the Data section of your Build an Atom Scientific Investigation Report.
- Use your cursor to add another neutron (silver) to the middle of the atom model. Complete the second row of the Build a Hydrogen Atom data table in the Data section of your Build an Atom Scientific Investigation Report.
- Use your cursor to add another neutron (silver) to the middle of the atom model. Complete the third row of the Build a Hydrogen Atom data table in the Data section of your Build an Atom Scientific Investigation Report.
- Use your cursor to add another electron (blue) to the atom model. Complete the fourth row of the Build a Hydrogen Atom data table in the Data section of your Build an Atom Scientific Investigation Report. Record the location of this electron in the notes section of the fourth row.
- Use your cursor to add another electron (blue) to the atom model. Complete the fifth row of the Build a Hydrogen Atom data table in the Data section of your Build an Atom Scientific Investigation Report. Record the location of this electron in the notes section of the fifth row.
- In the Build a Hydrogen Atom area of the Data section of your Build an Atom Scientific Investigation Report, draw an image of the atom that resulted from completing Steps 2-6.
Build a Hydrogen Atom Symbol
- On the bottom of your browser window, click on the icon for Symbol.
- From the "baskets" provided on the bottom of the screen, use your cursor to drop one proton (red) and one neutron (silver) in the middle of the atom model. Then, use your cursor to drop one electron (blue) onto the atom.
- Use your cursor to add two more neutrons (silver) to the middle of the atom model.
- Use your cursor to add two more electrons (blue) to the atom model.
- Record the symbol shown by the simulation in the Build a Hydrogen Symbol area in the Data section of your Build an Atom Scientific Investigation Report. Label what each number and letter indicates.
Build a Mystery Atom
- Repeat the Simulation Set-Up section of the Build an Atom Scientific Investigation.
- From the "baskets" provided on the bottom of the screen, use your cursor to drop any number of protons (red) and any number of neutrons (silver) in the middle of the atom model. Then, use your cursor to drop any number of electrons (blue) onto the atom. Complete the first row of the Build a Mystery Atom data table in the Data section of your Build an Atom Scientific Investigation Report.
- Use your cursor to add another proton (red) to the atom model. Complete the second row of the Build a Mystery Atom data table in the Data section of your Build an Atom Scientific Investigation Report.
- Use your cursor to add another electron (blue) to the atom model. Complete the third row of the Build a Mystery Atom data table in the Data section of your Build an Atom Scientific Investigation Report. Record the location of this electron in the notes section of the third row.
- In the Data section of your Build an Atom Scientific Investigation Report, draw or design an image of the atom that resulted from completing Steps 2-4.
Data
An excerpt from the Data section of the Build an Atom Scientific Investigation Report is found below. Please make sure to complete all tables and requested data on the report itself.
# of Protons |
# of Neutrons |
# of Electrons |
Name of Element |
Net Charge |
Mass Number |
Notes |
Data Analysis
In the Data Analysis section of your Build an Atom Scientific Investigation Report, provide responses to the following questions:
- What happens when an atom gains or loses electrons?
- How does a change in the number of protons compare to a change in the number of electrons? Do these changes have the same impact on an atom?
- What is an atom called when the number of neutrons changes? How does this impact the charge of the atom?
Conclusion
Using the Conclusion section of your Build an Atom Scientific Investigation Report, compose three to four sentences describing an overall conclusion about the relationship among protons, electrons, and neutrons, especially if any of the particles are added or subtracted from the atom. Were your hypotheses true or false, and how do you know? Use the data and notes that you collected from your simulation experience to form your conclusion. Make sure that you include information that you gained from data analysis to support your conclusion.
Experimental Sources of Error
On your Build an Atom Scientific Investigation Report, provide responses to the following questions: Are there any sources of error? If so, what are they, and what could be done to minimize error?
![]() Once you have completed the Build an Atom Scientific Investigation Report, please submit your work to the dropbox.
Once you have completed the Build an Atom Scientific Investigation Report, please submit your work to the dropbox.



