
Atoms - The Basics
Astronomers cannot go out to collect samples of stars to analyze them; however, they can use light from the stars to understand the nature of them. Atoms are the key to understanding this concept. Atoms are the tiny building blocks from which all matter is based upon.
These atoms make up the elements, which are pure chemical substances. These elements are arranged on the periodic table by their atomic numbers, which is the number of protons in each element. All atoms of the same element have the same number of protons. For instance, every hydrogen atom has one proton. For neutral atoms, the atomic number also tells how many electrons are in the atom because for each proton there is an electron. The hydrogen atom, therefore, has one electron. The periodic table also gives the symbol and sometimes the name of the element, as well as the atomic mass, which is weighted average of
that element’s naturally occurring isotopes. An element can have variants as determined by the existence of different numbers of neutrons in the atom. For example, the hydrogen atom can have no neutrons, two neutrons in a deuterium, and three neutrons in a tritium, to name a few.
Atomic Structure
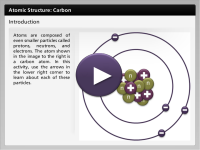 Atoms are composed of even smaller particles called protons, neutrons, and electrons. In this activity, use the arrows in the lower right corner to learn about each of these particles. The atom modeled in this interactivity is a carbon atom.
Atoms are composed of even smaller particles called protons, neutrons, and electrons. In this activity, use the arrows in the lower right corner to learn about each of these particles. The atom modeled in this interactivity is a carbon atom.
Download a printable version of the interactivity.
![]() To learn more about the history behind the discovery of the parts of the atom, view the video 1897 - First Subatomic Particle Found: The Electron from eMediaVA℠. As you view the video, make note of the order in which the different particles were discovered and how each was first noticed.
To learn more about the history behind the discovery of the parts of the atom, view the video 1897 - First Subatomic Particle Found: The Electron from eMediaVA℠. As you view the video, make note of the order in which the different particles were discovered and how each was first noticed.
Energy Changes and the Electron
The energy emitted or absorbed by the atom is associated with changes in the motion of the orbiting electron. Electrons can change their orbit when they receive a burst of energy. In 1913, Niels Bohr created the first model of an atom in which a positively charged nucleus was surrounded by electrons spinning on orbitals. Known as the Bohr model, it showed these electron shells around the nucleus.
 Bohr explained that the electrons were found at specific distances from the nucleus in what he called energy levels. His model helped to explain why the electrons did not crash into the positive nucleus. The electrons were moving with sufficient energy to maintain a position on the energy level. In this interactivity, click the arrows in the lower right corner to explore a Bohr model of the hydrogen atom and see how an atom can emit light just by changes in the energy of the electron.
Bohr explained that the electrons were found at specific distances from the nucleus in what he called energy levels. His model helped to explain why the electrons did not crash into the positive nucleus. The electrons were moving with sufficient energy to maintain a position on the energy level. In this interactivity, click the arrows in the lower right corner to explore a Bohr model of the hydrogen atom and see how an atom can emit light just by changes in the energy of the electron.
Download a printable version of the interactivity above.
![]() Learn how astronomers can use these facts about electrons to study celestial objects by viewing the video What Makes Light and Color in eMediaVA℠. In the video, you will see how light results from the energy of electron movements in different elements.
Learn how astronomers can use these facts about electrons to study celestial objects by viewing the video What Makes Light and Color in eMediaVA℠. In the video, you will see how light results from the energy of electron movements in different elements.
The Atom Review

![]() Check to see if you know the parts of the atom and what happens when the energy of an atom changes in this non-graded activity. Read each question and select the appropriate answer. Then, click SUBMIT to check your response. Click the interactivity thumbnail, and then click NEXT to begin.
Check to see if you know the parts of the atom and what happens when the energy of an atom changes in this non-graded activity. Read each question and select the appropriate answer. Then, click SUBMIT to check your response. Click the interactivity thumbnail, and then click NEXT to begin.


