
pH and pOH
Acids and Bases
Acids are substances that deliver the hydrogen ion (H+) to a solution. Bases are substances that deliver the hydroxide (OH-) ion to a solution, or cause water to produce hydroxide. It was Svante Arrhenius who first defined an acid and a base. Later, Nicolaus Bronsted and Thomas Martin Lowry improved upon the original definition.

Chemists Svante Arrhenius (left), Johannes Bronsted (center), and Thomas Lowry (right)
Arrhenius Theory
Svante Arrhenius was a Swedish chemist who published two articles on acids and bases. The first article was written in 1894, and the second was written in 1899. His articles defined both an acid and a base. These articles served as the foundation for his Acid Base Theory. What did the articles state? Arrhenius defined an acid as any substance that delivers the hydrogen ion (H+) to a solution. He described a base as any substance which delivers hydroxide ion (OH-) to a solution.
Bronsted-Lowry Definition
The Bronsted-Lowry Theory is an acid-base theory proposed by Johannes Nicolaus Bronsted and Thomas Martin Lowry in 1923. The main concept behind their theory is that acids donate a proton (H+), and that bases accept a proton. This theory is considered an improvement to the Arrhenius theory. In this theory, the bases do not have to contain hydroxide at all. They must cause water to produce hydroxide. How does it improve the Arrhenius method? Sodium hydroxide dissociates in water to create sodium ions and hydroxide ions. This satisfies the definition of a base in the Arrhenius definition.
NaOH → Na+ + OH-
Under Arrhenius' definition, ammonia, a very basic substance, would not be considered a base. Why? Ammonia does not fully dissociate in water. However, according the Bronsted-Lowry definition, ammonia is a basic substance because it accepts a proton. Ammonia takes a hydrogen anion from water to form ammonium ion and hydroxide ion.
NH3 + H2 → NH4+ +OH-
What is an Acid?
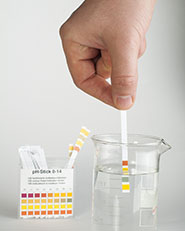
Litmus paper is used to test pH
What is an acid truly? An acid is a solution that has an excess of H+ ions. The more H+ ions a substance has, the more acidic the solution becomes. Properties of an acid include low pH, a sour taste, electrical conductivity, corrosion of metals, and turning blue litmus paper red. There are many uses of acids. Citric acid is found in fruits, such as lemons, limes, and oranges. Ascorbic acid is a key acid which your body needs to function. It is found in vitamin C. Sulfuric acid is used in the production of fertilizers, steel, paints, and plastics.
A base is a solution that has an excess of OH- ions. Bases are substances that can accept hydrogen ions. Properties of a base include; high pH, feeling slippery, tasting bitter, corroding metals, conducting electricity, not reacting with metals, and turning red litmus paper blue. There are also many uses to bases. The OH- ion in bases interacts with substances, such as dirt and grease. When bases are added to cleaning products, they are more efficient. One of the most important fluids in your body is basic: your blood.
How are Acids Measured?
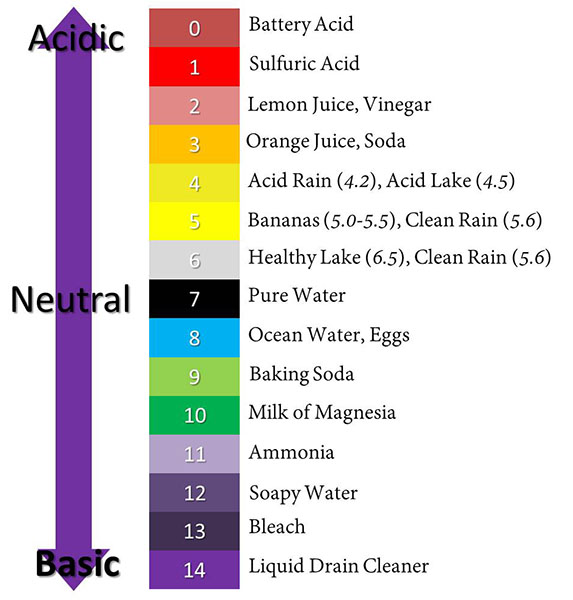
Hover your cursor over the pH Scale to view more details
Acids are measured using the pH scale. pH is a measure of how acidic or basic a solution is. The pH scale ranges from zero to fourteen. The pH number denotes the hydrogen (hydronium) ion concentration. The pOH denotes the hydroxide ion concentration. Solutions containing a higher concentration of hydronium H3O+ will have a lower pH. On the pH scale, acidic solutions have a pH value below seven. A solution with a pH of zero is very acidic. A solution with a pH of seven is neutral. Pure water has a pH of seven. Basic solutions have pH values above seven. A change of one pH unit represents a tenfold change in the acidity of the solution. For example, if one solution has a pH of one and a second solution has a pH of two, the first solution is not twice as acidic as the second; it is ten times more acidic.
Calculating pH
 The pH of a solution can be determined in the laboratory using litmus paper, but how is the pH of a solution calculated if you only know the molarity of the solution? In this interactivity, click on each of the bars in the "accordion" to learn more about calculating pH.
The pH of a solution can be determined in the laboratory using litmus paper, but how is the pH of a solution calculated if you only know the molarity of the solution? In this interactivity, click on each of the bars in the "accordion" to learn more about calculating pH.
View a printable version of the interactivity.
pH and pOH Review
![]()
 Now that you have explored pH and pOH, complete this non-graded activity to check your knowledge. Complete each of the pH calculations and use your cursor to select the correct pH on the pH scale. Then, click SUBMIT to check your responses. Click on the interactivity thumbnail, and then click NEXT to get started.
Now that you have explored pH and pOH, complete this non-graded activity to check your knowledge. Complete each of the pH calculations and use your cursor to select the correct pH on the pH scale. Then, click SUBMIT to check your responses. Click on the interactivity thumbnail, and then click NEXT to get started.


