
Naming of Acids
As you learned, acids are defined by both the Arrhenius definition and the Bronsted-Lowry definition. An acid is a molecular compound that contains one or more hydrogen atoms. When dissolved in water, the chemical bond between the hydrogen atom and the rest of the molecule breaks. This produces a hydrogen ion and anion.
There are also different types of acids. Binary acids consist of hydrogen and one other element. Oxoacids consist of hydrogen, oxygen, and a third element. Usually this third element is a nonmetal. A third type of acid is the organic acids. Organic acids are composed of an organic compound with some acidic properties.
 The naming of acids is based on the anion. Anions are either monatomic or polyatomic. All monatomic ions end with –ide. Polyatomic ions end in either –ate or –ite. In this activity, click the check boxes from the clipboard to examine the three rules used for naming an acid.
The naming of acids is based on the anion. Anions are either monatomic or polyatomic. All monatomic ions end with –ide. Polyatomic ions end in either –ate or –ite. In this activity, click the check boxes from the clipboard to examine the three rules used for naming an acid.
View a printable version of the interactivity.
Naming Acids Review

![]() Now that learned how to properly name acids, complete this non-graded activity to check your knowledge. Read each question and select the correct answer. Then, click SUBMIT to check your response. Click on the interactivity thumbnail, and then click NEXT to get started.
Now that learned how to properly name acids, complete this non-graded activity to check your knowledge. Read each question and select the correct answer. Then, click SUBMIT to check your response. Click on the interactivity thumbnail, and then click NEXT to get started.
Writing Formulas for Acids
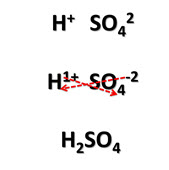 Acids are neutral compounds. The charge of the anion has to balance out the charge of the H+ ions. Since H+ ions carry a single positive charge, the number of H+ ions in the formula is equal to the quantity of the negative charge of the anion. In hydrochloric acid (HCl), there is one positive hydrogen ion. This is balanced out by one negative chloride ion. In sulfuric acid, H2SO4, there are two hydrogen atoms that are balanced out by the negative two charge of the sulfate ion. It will help to use the crisscross method to help write the correct chemical formula. Take a moment to review the criss-cross method in the image provided.
Acids are neutral compounds. The charge of the anion has to balance out the charge of the H+ ions. Since H+ ions carry a single positive charge, the number of H+ ions in the formula is equal to the quantity of the negative charge of the anion. In hydrochloric acid (HCl), there is one positive hydrogen ion. This is balanced out by one negative chloride ion. In sulfuric acid, H2SO4, there are two hydrogen atoms that are balanced out by the negative two charge of the sulfate ion. It will help to use the crisscross method to help write the correct chemical formula. Take a moment to review the criss-cross method in the image provided.
Writing Formulas for Acids Review

![]() Now that learned how to writing the formulas for acids, complete this non-graded activity to check your knowledge. Drag each of the formulas on the right and drop them next to the acid names on the left. Once you have matched all of the formulas with the correct acid, select SUBMIT to check your answers. Click on the interactivity thumbnail, and then click NEXT to get started.
Now that learned how to writing the formulas for acids, complete this non-graded activity to check your knowledge. Drag each of the formulas on the right and drop them next to the acid names on the left. Once you have matched all of the formulas with the correct acid, select SUBMIT to check your answers. Click on the interactivity thumbnail, and then click NEXT to get started.


