
Naming of Acids
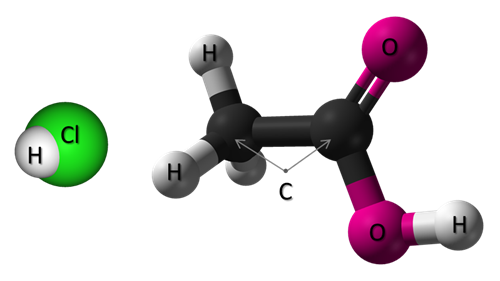
Hydrochloric Acid (HCl) and Acetic Acid (C2H4O2)
Scientists must communicate information carefully and consistently. The naming system you just studied allows for a distinct name for every acid. The anion and the presence and quantity of hydrogen ions are used in determining the name of the acid. For example, an anion with a negative two charge will combine with two hydrogen cations. This occurs because compounds do not have charges. The ions that make up the compounds must combine in a way to cancel out the positive charges and negative charges. The anion involved in an acid will also greatly affect the acid strength. The difference between hydrochloric acid and citric acid is huge when it comes to their relative strengths and toxicity. While both are acids, the differing anions result in different levels of dissociation.




