
Parts of the Atom and Isotope Symbol
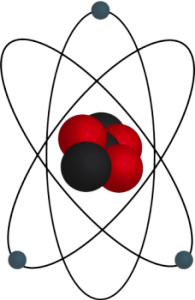 There are tiny particles called atoms. These particles are much smaller than the human eye can see. These tiny particles are composed of even smaller particles - protons, neutrons, and electrons. The total mass of the neutrons and protons in an atom is the mass number. It is possible for atoms of the same element to have a different number of neutrons and therefore, a different mass. They are called isotopes.
There are tiny particles called atoms. These particles are much smaller than the human eye can see. These tiny particles are composed of even smaller particles - protons, neutrons, and electrons. The total mass of the neutrons and protons in an atom is the mass number. It is possible for atoms of the same element to have a different number of neutrons and therefore, a different mass. They are called isotopes.
In order for an atom to be electrically neutral, there must be an equal number of protons and electrons. Electrons have a charge equal, but opposite to the proton. They are located outside of the nucleus in the electron cloud.




