Electron Configuration and Orbital Diagrams
 The periodic table can be thought of as a map for the configuration, or arrangement, of electron around the nucleus of an atom. Each period correlates to a specific energy level, and each group relates to a different sublevel. The principle quantum number, n, corresponds to the period on the periodic table. For example, elements in period one have one energy level, elements in period two have two energy levels, elements in period three have three energy levels, and so on. In this interactivity, click on each of the tabs to learn more about each of the sublevels on the periodic table.
The periodic table can be thought of as a map for the configuration, or arrangement, of electron around the nucleus of an atom. Each period correlates to a specific energy level, and each group relates to a different sublevel. The principle quantum number, n, corresponds to the period on the periodic table. For example, elements in period one have one energy level, elements in period two have two energy levels, elements in period three have three energy levels, and so on. In this interactivity, click on each of the tabs to learn more about each of the sublevels on the periodic table.
Download a printable version of the interactivity.
 Aufbau Principle/Diagonal Rule
Aufbau Principle/Diagonal Rule
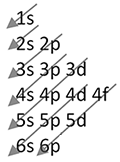
The electrons fill in a specific order, from lowest to highest energy. The d and f sublevels are less energy efficient than the s and p sublevels, and thus will fill later. As viewed in the image on the right, orbital 1s fills in first followed by 2s, then 2p, 3s, 3p, 4s, 4d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, 7s, 5f, and 6d.
Constructing Orbital Diagrams
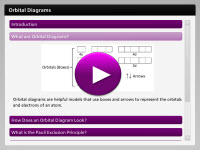 Orbital diagrams use a series of boxes and arrows used to represent the configuration of electrons within an atom. While these models cannot represent the exact location of the electrons, they still serve as a helpful visual map of the probable location of the electrons outside of an atoms nucleus. In this interactivity, click on each of the questions to learn how to complete an orbital diagram.
Orbital diagrams use a series of boxes and arrows used to represent the configuration of electrons within an atom. While these models cannot represent the exact location of the electrons, they still serve as a helpful visual map of the probable location of the electrons outside of an atoms nucleus. In this interactivity, click on each of the questions to learn how to complete an orbital diagram.
View a printable version of the interactivity.
Electron Configuration
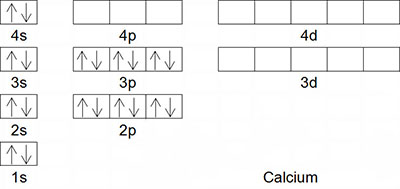
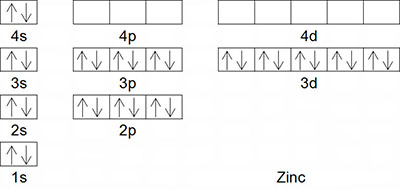
Like orbital diagrams, electron configurations are used to categorize the electrons in an atom. Instead of drawing all of the boxes and arrows, an electron configuration simply displays the energy level, sublevel and number of electrons. Earlier, you viewed the orbital diagram for the element calcium. This diagram is also shown on the right. Roll your cursor over the orbital diagram for calcium to see its electron configuration. Notice that the same information is displayed in an electron configuration but in a different way. The numbers, written as coefficients, represent the energy levels. This shows that calcium has electrons occupying the first four energy levels. The letters represent the sublevel. This shows that the s and p sublevels are occupied for calcium. Finally, the electrons are shown as exponents. This shows that the first energy level has two electrons, the second energy level has eight, the third energy level has eight and the fourth energy level has two electrons. Now, try to write the electron configuration for zinc and then hover your mouse over the orbital diagram for zinc to see its electron configuration.
Abbreviated Electron Configuration
As you create electron configurations, you may notice that some become very long. If this is the case, an abbreviated or condensed electron configuration is used. The abbreviated electron configuration is a shorter, more concise method. For example, calcium's electron configuration is 1s22s22p63s23p64s2. This is shortened or abbreviated using the element argon. Here is calcium's abbreviated electron configuration: [Ar] 4s2. Argon is used to abbreviate because it has the same starting configuration as calcium; 1s22s22p63s23p6.
Valence Electrons and Oxidation
 Reactivity is the ability of an element to enter into a chemical reaction, and a valence electron is an electron that can participate in the formation of chemical bonds. View this presentation to learn more about valence electrons and how to determine the ability or tendency of an atom to enter into a bond. Make sure to take note of how to determine the number of valence electrons in an atom.
Reactivity is the ability of an element to enter into a chemical reaction, and a valence electron is an electron that can participate in the formation of chemical bonds. View this presentation to learn more about valence electrons and how to determine the ability or tendency of an atom to enter into a bond. Make sure to take note of how to determine the number of valence electrons in an atom.
View a printable version of the interactivity.
![]() To learn why some groups of elements are more likely to react that others, view the video What Makes an Element Reactive? from eMediaVA℠. As you view the video, take note of how the electron configuration of an atom affects its ability to combine with other atoms.
To learn why some groups of elements are more likely to react that others, view the video What Makes an Element Reactive? from eMediaVA℠. As you view the video, take note of how the electron configuration of an atom affects its ability to combine with other atoms.
Electron Configuration and Orbital Diagrams Review
![]() Now that you have learned about orbital diagrams, electron configurations, valence electrons, and oxidation check your knowledge in this non-graded activity. Read the instructions provided with each question and select or provide the correct answer. Then, click SUBMIT to check your response. Click on the interactivity thumbnail, and then click NEXT to get started.
Now that you have learned about orbital diagrams, electron configurations, valence electrons, and oxidation check your knowledge in this non-graded activity. Read the instructions provided with each question and select or provide the correct answer. Then, click SUBMIT to check your response. Click on the interactivity thumbnail, and then click NEXT to get started.