
Electron Configuration and Orbital Diagrams
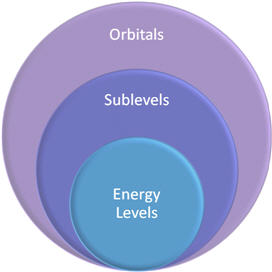 The Bohr model of the atom left some unanswered questions regarding the electrons. Bohr established that electrons are not just outside the nucleus, but on specific energy levels a certain distance from the nucleus. This still did not explain the position of electrons accurately. While the exact position of an electron cannot be established, quantum mechanics provides a model that shows the an electron's most probable position. The energy levels are divided into sublevels and these sublevels into orbitals. In this topic, you will investigate how electron configurations relate to the periodic table. You will also apply the Aufbau Principle, the Pauli Exclusion Principle, and Hund’s Rule to electron configuration.
The Bohr model of the atom left some unanswered questions regarding the electrons. Bohr established that electrons are not just outside the nucleus, but on specific energy levels a certain distance from the nucleus. This still did not explain the position of electrons accurately. While the exact position of an electron cannot be established, quantum mechanics provides a model that shows the an electron's most probable position. The energy levels are divided into sublevels and these sublevels into orbitals. In this topic, you will investigate how electron configurations relate to the periodic table. You will also apply the Aufbau Principle, the Pauli Exclusion Principle, and Hund’s Rule to electron configuration.
Essential Questions
- How are orbital diagrams constructed and analyzed?
- What is electron configuration and how is it used to show the organization of electrons in an atom?