
Naming Molecular Compounds
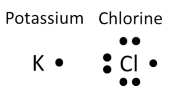
![]() Before you learn how to name molecular compounds, you need to review how the sharing of electrons takes place. In this Warm-Up, consider the bonding of potassium and chlorine. Do you remember the Lewis dot diagrams for each of these elements? Take a moment and review the Lewis dot diagrams for both potassium and chlorine.
Before you learn how to name molecular compounds, you need to review how the sharing of electrons takes place. In this Warm-Up, consider the bonding of potassium and chlorine. Do you remember the Lewis dot diagrams for each of these elements? Take a moment and review the Lewis dot diagrams for both potassium and chlorine.
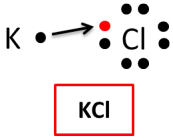 Now that you have examined the Lewis dot diagrams, how many electrons does chlorine need to have a stable octet? The answer is one. Clearly, when these two elements form a compound, potassium will transfer its one valence electron to chlorine, which needs one valence electron. The bond created
Now that you have examined the Lewis dot diagrams, how many electrons does chlorine need to have a stable octet? The answer is one. Clearly, when these two elements form a compound, potassium will transfer its one valence electron to chlorine, which needs one valence electron. The bond created 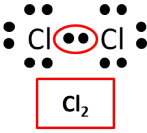 is an ionic bond. The end result is the compound potassium chloride.
is an ionic bond. The end result is the compound potassium chloride.
Now consider a case in which two chlorine atoms bond. Once again review the Lewis dot diagrams for a chlorine atom bonding with another chlorine atom. In this case, the atoms share an electron because chlorine only needs one electron to have a stable octet. The bond created is a covalent bond.
![]() Remember, atoms want to stabilize by gaining, losing, or sharing electrons. The dangerous metal sodium proves that atoms want to stabilize. Learn more about sodium in the video clip How Elements Form Compounds from eMediaVA℠ .
Remember, atoms want to stabilize by gaining, losing, or sharing electrons. The dangerous metal sodium proves that atoms want to stabilize. Learn more about sodium in the video clip How Elements Form Compounds from eMediaVA℠ .

