
Percent Composition
Percent Composition is SWEET! Scientific Investigation

![]() Before you begin the scientific investigation below, make sure to download the Percent Composition is SWEET! Scientific Investigation Report. As you complete this scientific investigation, fill in any needed information on the report template. If you need more information about each section of the report, please visit the Developmental Module.
Before you begin the scientific investigation below, make sure to download the Percent Composition is SWEET! Scientific Investigation Report. As you complete this scientific investigation, fill in any needed information on the report template. If you need more information about each section of the report, please visit the Developmental Module.
This scientific investigation is available below or in a printable version.
Introduction
Elements combine to make compounds by bonding. It is possible for elements to combine in multiple ratios. This is known as the Law of Multiple Proportions. Because of this, these various compounds will have different percent composition. In this activity you will be analyzing a certain compound named cookium (a cookie) that consists of three atoms all together - two “wafer” atoms sandwiching one “cream” atom.
Objectives
In this scientific investigation, you will:
- develop a mathematical model of percent composition from experimental data;
- relate slope to different chemical quantities used in chemistry; and
- make predictions based on a model.
Hypothesis
Using the Procedure and Data Collection section below, read through the procedural information for this scientific investigation. Based on your understanding of the procedure, develop your own hypotheses which describe your expected results. Specifically, how do you think the ink of each marker will behave when chromatography is performed? Record these hypotheses in the Hypothesis section of your Percent Composition is SWEET! Scientific Investigation Report.
Equipment and Materials
- 16 sandwich cookies
- Plastic gloves
- Electronic or triple beam balance
- Percent Composition with Cookies Excelet
- 4 plastic knives
- 4 paper plates
Procedure and Data Collection
Part 1
- Zero the balance with one of the paper plates on it. Add one cookie to the plate and record the mass of the cookie in the Data section of your Percent Composition is SWEET! Scientific Investigation Report. Record the mass to the nearest 0.1 gram. Remove the cookie from the balance.
- Scrape the cream off of the same cookie, and consume or dispose of the cream. Add both of the wafers to the plate and record the mass of the two wafers in the Data section of your Percent Composition is SWEET! Scientific Investigation Report. Record the mass to the nearest 0.1 gram.
Part 2
- Zero the balance with one of the paper plates on it. Add three cookies to the plate and record the mass of the three cookies in the Data section of your Percent Composition is SWEET! Scientific Investigation Report. Record the mass to the nearest 0.1 gram. Remove the three cookies from the balance.
- Scrape the cream off of the same three cookies, and consume or dispose of the cream. Add all six of the wafers to the plate and record the mass of the six wafers in the Data section of your Percent Composition is SWEET! Scientific Investigation Report. Record the mass to the nearest 0.1 gram.
Part 3
- Zero the balance with one of the paper plates on it. Add five cookies to the plate and record the mass of the five cookies in the Data section of your Percent Composition is SWEET! Scientific Investigation Report. Record the mass to the nearest 0.1 gram. Remove the five cookies from the balance.
- Scrape the cream off of the same five cookies, and consume or dispose of the cream. Add all ten of the wafers to the plate and record the mass of the ten wafers in the Data section of your Percent Composition is SWEET! Scientific Investigation Report. Record the mass to the nearest 0.1 gram.
Part 4
- Zero the balance with one of the paper plates on it. Add seven cookies to the plate and record the mass of the seven cookies in the Data section of your Percent Composition is SWEET! Scientific Investigation Report. Record the mass to the nearest 0.1 gram. Remove the seven cookies from the balance.
- Scrape the cream off of the same seven cookies, and consume or dispose of the cream. Add all fourteen of the wafers to the plate and record the mass of the fourteen wafers in the Data section of your Percent Composition is SWEET! Scientific Investigation Report. Record the mass to the nearest 0.1 gram.
Part 5
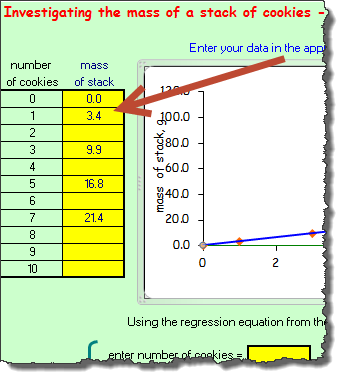 Now that you have the mass of the whole cookies, as well as the mass of the wafers, calculate the mass of the cream for one, three, five, and seven cookies. Place this information in the fourth column of the data table in the Data section of your Percent Composition is SWEET! Scientific Investigation Report.
Now that you have the mass of the whole cookies, as well as the mass of the wafers, calculate the mass of the cream for one, three, five, and seven cookies. Place this information in the fourth column of the data table in the Data section of your Percent Composition is SWEET! Scientific Investigation Report.- Open the Percent Composition with Cookies Excelet.
- On each of the tabs of the spreadsheet, add the number of cookies and the mass amount requested as shown in the image to the right. For the quantity of cookies that you did not measure (two, four, six, etc.), leave the row blank.
- As you complete the data table for each of the tabs, the graphs will populate with the data that you are providing. Based on the graph's data, provide the slope for each of the following and record it in the Data section of your Percent Composition is SWEET! Scientific Investigation Report:
- The mass of the cookie vs. the number of cookies
- The mass of the cream vs. the number of cookies
- The mass of the wafer vs. the number of cookies
- The mass of the cream vs. the mass
Save your completed Percent Composition with Cookies Excelet for submission with your Percent Composition is SWEET! Scientific Investigation Report.
Data
Use the area provided on your Percent Composition is SWEET! Scientific Investigation Report to record your data from this scientific investigation. The data tables are also shown below:
Mass Recording Data Table
| Number of Cookies | Mass of Cookies | Mass of Wafers | Mass of Cream |
| 1 | |||
| 3 | |||
| 5 | |||
| 7 |
Slope Recording Data Table
| Graph | Slope |
| The mass of the cookie vs. the number of cookies | |
| The mass of the cream vs. the number of cookies | |
| The mass of the wafer vs. the number of cookies | |
| The mass of the cream vs. the mass of the cookie |
Data Analysis
In the Data Analysis section of your Percent Composition is SWEET! Scientific Investigation Report, provide responses to the questions below. Make sure to show all of your calculations and to use the appropriate number of significant figures.
- What is the molar mass of the cookium compound?
- What is the molar mass of the cream?
- What is the molar mass of a single wafer?
- How much would a cookie that contained three wafers and two layers of cream weigh?
- How much would a cookie that had two wafers and two layers of cream weigh?
- What is the percent cream in a cookie that is composed of three wafers and two layers of cream?
- What is the percent cream in a cookie that is composed of two wafers and two layers of cream?
- For each tab on the Percent Composition with Cookies Excelet, what information does the slope of the line on your graph tell you about the relationship between the independent and dependent variable? Provide specific information about each graph.
- Now, apply this to a real chemical compound. What is the percent oxygen (O) in water H2O?
- What is the percent carbon (C) in calcium carbonate CaCO3?
- What is the percent cobalt (Co) in cobalt III sulfate Co2(SO4)3?
Conclusion
Using the Conclusion section of your Percent Composition is SWEET! Scientific Investigation Report, compose three to four sentences describing an overall conclusion based on your data. Were your hypotheses true or false, and how do you know? Use the data and notes that you collected from your investigation to form your conclusion. Make sure that you include information that you gained from data analysis to support your conclusion.
Experimental Sources of Error
On your Percent Composition is SWEET! Scientific Investigation Report, provide responses to the following questions: Are there any sources of error? If so, what are they, and what could be done to minimize error?
![]() Once you have completed the Percent Composition is SWEET! Scientific Investigation Report and the Percent Composition with Cookies Excelet, please submit your work to the dropbox.
Once you have completed the Percent Composition is SWEET! Scientific Investigation Report and the Percent Composition with Cookies Excelet, please submit your work to the dropbox.



