
Percent Yield
Part A: Percent Yield Calculations

![]() One of the most important breakthroughs in the study of chemistry resulted from the work of Antoine Lavoisier. He was one of the first chemists to realize the importance of careful measurements of all substances in a reaction. In fact, he was so interested in careful measurements that he invented his own balance which was correct to within 0.0005 grams. His careful measurements led to the finding that the mass of the reactants must always equal the mass of the products. His discovery of the Law of Conservation of Mass is why we now refer to him as the Father of Modern Chemistry.
One of the most important breakthroughs in the study of chemistry resulted from the work of Antoine Lavoisier. He was one of the first chemists to realize the importance of careful measurements of all substances in a reaction. In fact, he was so interested in careful measurements that he invented his own balance which was correct to within 0.0005 grams. His careful measurements led to the finding that the mass of the reactants must always equal the mass of the products. His discovery of the Law of Conservation of Mass is why we now refer to him as the Father of Modern Chemistry.
Some of Lavoisier’s most important measurements involved combustion reactions. One of the reactions he performed and measured was the reaction of hydrogen and oxygen to form water: 2H2 + O2→ 2H2O. Use the information from this topic to answer the questions below. Make sure to show all of your work.
This activity is available below or in a printable version.
- If Lavoisier reacted 168 liters of hydrogen at STP and produced 13.5 grams of water, what was his percent yield?
- 35.0 liters of oxygen was reacted with excess hydrogen to produce 48.0 grams of water. What is the percent yield?
- 79.0 grams of water was produced with a percent yield of 75.0%. How many grams of oxygen did he start with?
- 2.00 moles of oxygen gas at STP will produce how many grams of water, if the percent yield is 82.0%?
![]() Once you have completed the mole conversions using dimensional analysis, please submit your work to the dropbox.
Once you have completed the mole conversions using dimensional analysis, please submit your work to the dropbox.
Part B: Synthesis of Aspirin Scientific Investigation
![]()
![]() Before you begin this assignment, make sure to download the Synthesis of Aspirin Scientific Investigation Report. As you complete this scientific investigation, fill in any needed information on the report template. If you need more information about each section of the report, please visit the Developmental Module.
Before you begin this assignment, make sure to download the Synthesis of Aspirin Scientific Investigation Report. As you complete this scientific investigation, fill in any needed information on the report template. If you need more information about each section of the report, please visit the Developmental Module.
This scientific investigation is available below or in a printable version.
Introduction
Aspirin is a widely used medicinal drug. Aspirin is a naturally occurring compound that can be derived from the bark of the willow trees. The actual compound is named acetylsalicylic acid. The willow trees use to relieve pain can be traced back to the early Native Americans. When in pain, Native Americans would chew the bark or grind it into a powder for ingestion. Of course, obtaining pain relief by grinding the bark of a willow tree is not practical in modern times. An easier solution is to produce acetylsalicylic acid in a laboratory setting. Synthesizing acetylsalicylic is more feasible and cost-effective, while producing a product of equal quality. Below you can view the chemical structure for aspirin.
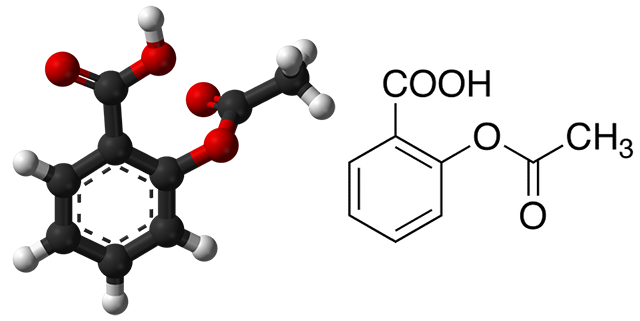
Objectives
In this scientific activity, you will:
- synthesize aspirin by reacting salicylic acid with acetic anhydride;
- calculate the theoretical yield of aspirin in grams; and
- calculate the percent yield of the aspirin synthesis reaction.
Hypothesis
Using the Procedure and Data Collection section below, read through the procedural information for this scientific investigation. Based on your understanding of the procedure, develop your own hypotheses which describe your expected results. Specifically, will aspirin be synthesized by reacting salicylic acid with acetic anhydride? Record these hypotheses in the Hypothesis section of your Synthesis of Aspirin Scientific Investigation Report.
Equipment and Materials
- Salicylic Acid
- Acetic Anhydride
- 85% Phosphoric Acid (concentrated)
- Gloves
- Safety Goggles
- 250 Erlenmeyer Flask
- 10 mL graduated cylinder
- 25 mL graduated cylinder
- Filter Paper
- Buchner Funnel
- Stirring Rod
- Digital Scale of Triple-beam Balance
- Watch Glass
- 1 mL Pipette
- Fume Hood
- Oven
- 2 Large Beakers (500 mL or larger)
- Distilled Water
- Ice
- Water aspirator
Procedure
It is important to follow all safety guidelines and procedures with this laboratory activity. This experiment uses salicylic acid, acetic anhydride, and phosphoric acid. Both salicylic acid and aspirin may cause irritation to your skin or eyes. Both of these compounds can be disposed of in the sink. If either of these two compounds accidentally spill, wipe them up with a wet paper towel and dispose of the towel in the trash. Acetic anhydride and phosphoric acid can cause severe burns. These chemicals must be used under a fume hood. When handling these chemicals, safety goggles and gloves must be worn at all times. If these chemicals exist in excess, the remaining chemical should be disposed of in a plastic tub of water. The water will dilute phosphoric acid and turn the acetic anhydride to vinegar. If either of these chemicals accidentally spill, notify your instructor immediately.
- Using the digital scale or triple-beam balance, measure 3.0 grams of salicylic acid. Place this acid in a 250 mL Erlenmeyer flask.
- In the fume hood, add 6.0 mL acetic anhydride to a 10 mL graduated cylinder. Pour the contents of the graduated cylinder into the Erlenmeyer flask that contains the salicylic acid.
- Remaining in the fume hood, carefully add 5 to 10 drops of 85% phosphoric acid. Swirl the flask to ensure that the chemicals are mixed thoroughly.
- Still in the fume hood, fill a larger breaker with warm water at a temperature of 70-80°C. Place the Erlenmeyer flask inside of the beaker of water and let it sit for ten minutes.
- After the ten minutes has ended, carefully add 20 mL of distilled water to the mixture using the 25 mL graduated cylinder.
- At your laboratory station, fill a large beaker with ice and water. Remove your Erlenmeyer flask from the fume hood and place the flask in the ice bath. Allow enough time for the crystallization of your mixture. If crystallization is not occurring, use your stirring rod to scrap the sides of your Erlenmeyer flask to aid in the formation of crystals.
- Filter the solid aspirin through a piece of pre-weighted filter paper using a Buchner funnel and water aspirator. Wash the crystals with 2-3 ml of ice cold water. The liquid is mostly water and can be allowed to drain down the sink or into an additional Erlenmeyer flask.
- After the aspirin is washed, allow the aspirin and filter paper to air dry for 15 minutes. Record the mass of the filter paper mass.
- Place the filter paper with aspirin on a watch glass and put into an oven at 100°C. Allow the watch glass, filter paper, and aspirin to dry in the oven for 30 minutes, or until dry.
- Weigh the dry aspirin and filter paper, record the mass.
- Calculate the weight of your product by subtracting the mass of the paper from the mass of the filter paper and aspirin.
Data
Use the Data area provided on your Synthesis of Aspirin Scientific Investigation Report to record any observations and data you had while you conducting the procedure. A sample of the required data table is shown below.
| Mass of filter paper | g |
| Mass of filter paper with aspirin | g |
| Mass of aspirin | g |
Data Analysis
In the Data Analysis section of your Synthesis of Aspirin Scientific Investigation Report, provide the responses to the following questions:
- Describe the appearance of your final product.
- The molar ratio of salicylic acid to aspirin is 1:1. If you used 3.0 grams of salicylic acid in this experiment, calculate the theoretical yield of aspirin in grams.
- What is the percent yield of your aspirin synthesis reaction?
Conclusion
Using the Conclusion section of your Synthesis of Aspirin Scientific Investigation Report, compose three to four sentences describing an overall conclusion about synthesis of aspirin. Base your conclusions on your data. Were your hypotheses true or false, how do you know? Use the data and notes that you collected from your experiment to form your conclusion. Make sure that your include information that your gained from data analysis to support your conclusion.
Experimental Sources of Error
On your Synthesis of Aspirin Scientific Investigation Report, provide responses to the following questions: Are there any sources of error? If so, what are they, and what could be done to minimize error?
![]() Once you have completed your Synthesis of Aspirin Scientific Investigation Report, please submit your work to the dropbox.
Once you have completed your Synthesis of Aspirin Scientific Investigation Report, please submit your work to the dropbox.



