
Dalton's Law
Earth is sometimes called the “Goldilocks” planet. The temperature, pressure, and other conditions are just right for living. Our sister planet Venus is much different. Venus has an atmosphere that is much thicker than our planet. The compositions of the gases that make up Venus are also different. The majority of Venus is carbon dioxide and nitrogen. With a different percentage of gases, Venus has a much higher atmospheric pressure. How high is the pressure? On Venus the pressure can reach over 2700 mm Hg. This also creates a temperature above 460°C.
 English Chemist John Dalton studied the mixture of gases. Gas pressure is created when the gas particles collide against the inside walls of the container. Of course, if more gas is added to the same container, the pressure will increase as more collisions take place. What Dalton discovered was that each gas in a container exerts a pressure that is independent of the other gases in a mixture. If there are two gases in a container, then the sum of those two gas’s pressures is equal to the total pressure. Relating this back to Earth’s atmosphere, you will find that seventy-eight percent of the atmosphere is nitrogen and twenty-one percent is oxygen. Since the atmospheric pressure on Earth is equal to 1 atm, nitrogen makes up 0.78 percent of the atmospheric pressure, oxygen makes up 0.21 percent of the atmospheric pressure, and the other trace gases make up the final 0.01 percent.
English Chemist John Dalton studied the mixture of gases. Gas pressure is created when the gas particles collide against the inside walls of the container. Of course, if more gas is added to the same container, the pressure will increase as more collisions take place. What Dalton discovered was that each gas in a container exerts a pressure that is independent of the other gases in a mixture. If there are two gases in a container, then the sum of those two gas’s pressures is equal to the total pressure. Relating this back to Earth’s atmosphere, you will find that seventy-eight percent of the atmosphere is nitrogen and twenty-one percent is oxygen. Since the atmospheric pressure on Earth is equal to 1 atm, nitrogen makes up 0.78 percent of the atmospheric pressure, oxygen makes up 0.21 percent of the atmospheric pressure, and the other trace gases make up the final 0.01 percent.
Dalton’s study of the contained gases led to the Law of Partial Pressures. This law states that the total pressure exerted by a mixture of gases is equal to the sum of the partial pressures of all contained gases. In this law, the partial pressure is indicated by P with subscript for each of the different gases. The mathematical expression for the Law of Partial Pressures is:
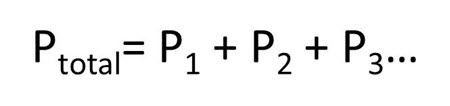
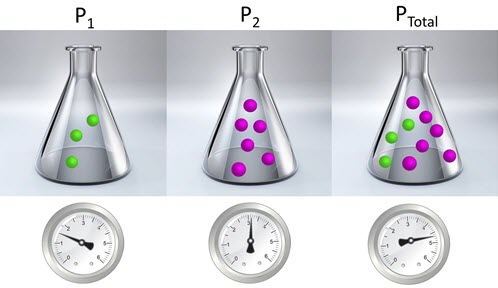
In the image below, you can view two gases in equal, separate containers. Imagine that these gases are under the same temperature and pressure. You can see that the pressure of P1 is less than P2. P1 has a pressure of 1.5 atm. P2 has a pressure of 3.2. This means that if the gases are placed in the same container the total pressure of the system (Ptotal) is equal to 4.7 atm.
Law of Partial Pressures Review
![]()
 Now that you have explored Dalton’s Law of Partial Pressures, review your knowledge in this non-graded activity. Read each problem, list the unknown and known variables, and solve the problem. Once you have completed the problem, select the correct answer. Click SUBMIT to check your responses. Click on the interactivity thumbnail, and then click NEXT to get started.
Now that you have explored Dalton’s Law of Partial Pressures, review your knowledge in this non-graded activity. Read each problem, list the unknown and known variables, and solve the problem. Once you have completed the problem, select the correct answer. Click SUBMIT to check your responses. Click on the interactivity thumbnail, and then click NEXT to get started.


