
Radiometric Dating
Radiometric dating is a method of finding the precise numerical age of rocks or fossils. The process of radiometric dating, also known as absolute dating, occurs in a laboratory with sophisticated instruments and computer technology. Radiometric dating requires an analysis of the rock's composition first. A rock is composed of a mixture of elements. When attempting to find the rock's precise age, scientists must find special atoms of elements present in the rock called isotopes. An isotope is an atom with an abnormal amount of neutrons present in the nucleus. This abnormal amount of neutrons will affect the atom's atomic weight, but will not alter the chemical identity of the element. The identity of the element is determined by the number of protons present in the nucleus.
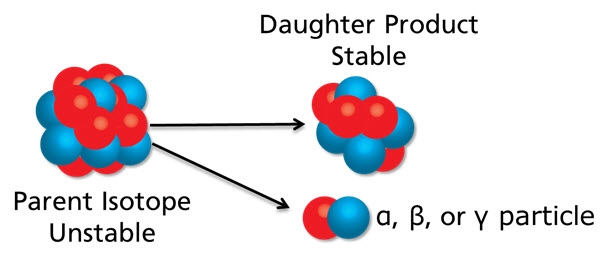
The Process of Radioactive Decay
As a result of having extra neutrons, the atom behaves abnormally and begins to decay. The radioactive isotope, or parent isotope, tries to shed the extra nuclear mass by releasing matter and energy from its nucleus to become more stable. This process is called radioactive decay. The process of radioactive decay occurs at a constant, precise rate called a half-life. As the parent isotope releases matter from its nucleus, it changes into a new, more stable element called the daughter isotope. The parent isotope also releases energy in the form of either alpha, beta, or gamma radiation. You can view radioactive decay in the image above. Since the decay process happens at a precise rate, it is possible to determine an age of the rock if you can detect the presence of these isotopes.
 Half-life is the rate at which a radioactive parent isotope decays to form a more stable daughter isotope. To be precise, half-life is the time required for half, or fifty percent, of the radioactive material to decay. Every radioactive isotope has a unique value for half-life. Some parent isotopes have very short half-lives, and other parent isotopes have very long half-lives. View this activity to learn more about the concept of half-lives. Click the player button to begin.
Half-life is the rate at which a radioactive parent isotope decays to form a more stable daughter isotope. To be precise, half-life is the time required for half, or fifty percent, of the radioactive material to decay. Every radioactive isotope has a unique value for half-life. Some parent isotopes have very short half-lives, and other parent isotopes have very long half-lives. View this activity to learn more about the concept of half-lives. Click the player button to begin.
View a printable version of the interactivity.
Common Isotopes
Parent Isotope |
Daughter Isotope |
Half-life |
Uranium-238 |
Lead-206 |
4.5 billion years |
Uranium-235 |
Lead-207 |
713 million years |
Throium-232 |
Lead-208 |
14.1 billion years |
Rubidium-97 |
Strontium-87 |
47.0 billion years |
Carbon-14 |
Nitrogen-14 |
5,730 years |
Potassium-40 |
Argon-40 |
1.3 billion years |
The table shown above identifies common radioactive parent isotopes, their known half-life value, and their resulting daughter isotopes. Each parent isotope is identified by its elemental name and a number. The name of the element indicates how many protons it contains according to the periodic table. The number next to the name indicates its atomic mass. Since parent isotopes have irregular atomic masses, they will decay at a constant rate indicated by the half-life value. Regardless of the half-life value, a parent isotope’s half-life value always remains constant. The rate does not speed up or slow down. This constant rate of decay is the reason why these atoms are so helpful in determining the age of a mineral, rock, or fossil.
Radiocarbon Dating
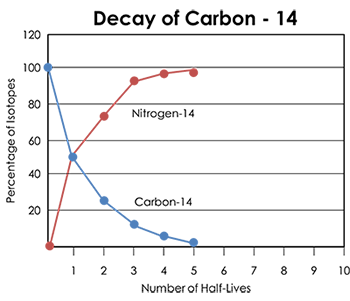 Radiocarbon dating is used to find the age of once-living materials between 100 and 50,000 years old. This range is especially useful for determining the ages of human fossils and habitation sites. An organism naturally collects carbon-14 throughout its lifetime. Once the organism dies, it stops collecting carbon-14. The carbon-14 has a half-life of 5,730 years. Every 5,730 years, only half of the original carbon-14 will remain. The daughter product for carbon-14 is nitrogen-14.
Radiocarbon dating is used to find the age of once-living materials between 100 and 50,000 years old. This range is especially useful for determining the ages of human fossils and habitation sites. An organism naturally collects carbon-14 throughout its lifetime. Once the organism dies, it stops collecting carbon-14. The carbon-14 has a half-life of 5,730 years. Every 5,730 years, only half of the original carbon-14 will remain. The daughter product for carbon-14 is nitrogen-14.
After the organism’s death, carbon-14 decays to stable nitrogen-14 by releasing a beta particle. The nitrogen atoms are lost to the atmosphere, but the amount of carbon-14 that has decayed can be estimated by measuring the proportion of radioactive carbon-14 to stable carbon-12. As shown by the graph, the amount of carbon-14 decreases relative to the amount of nitrogen-14. Scientists can use this ratio to find an accurate age of the organism.
Limitations
 Radiometric dating does have a few limitations. While this process is great for many igneous and metamorphic rocks, sedimentary rocks are made of fragments of other rocks. Each fragment would have a separate absolute age. In order to date sedimentary rocks, scientists must find and date igneous or metamorphic rocks found near the sedimentary rock. The absolute age of nearby rocks can help provide a relative age for the sedimentary rock layers.
Radiometric dating does have a few limitations. While this process is great for many igneous and metamorphic rocks, sedimentary rocks are made of fragments of other rocks. Each fragment would have a separate absolute age. In order to date sedimentary rocks, scientists must find and date igneous or metamorphic rocks found near the sedimentary rock. The absolute age of nearby rocks can help provide a relative age for the sedimentary rock layers.
Accurate absolute ages can only be applied when the parent rock has not experienced any loss. For example, potassium-40 has a half-life of 1.3 billion years. The daughter product is argon-40. To calculate the age using potassium and argon, scientists can compare the ratio of unstable potassium to stable potassium in the sample. Since the daughter product is a gas, it is possible that the gas may escape into the atmosphere. This would throw off any measurements.
Radiometric Dating Review

![]() Now that you have investigated the radiometric dating, review your knowledge. In this non-graded interactivity, read each question and select the appropriate answer. Click the player button to get started.
Now that you have investigated the radiometric dating, review your knowledge. In this non-graded interactivity, read each question and select the appropriate answer. Click the player button to get started.


