Wave-Particle Duality
![]() You will see in this video clip, The Nature of Light from Discovery Education™ streaming, that understanding the dual nature of light was challenging to physicists. Our understanding of light developed over a long period of time, from the time of Isaac Newton, the late 1600’s to the Einstein’s time, the early 1900’s. Today we know that light has a dual nature – it can behave like a particle or a wave. Any experiment using light will illustrate one side of the nature of light but not both at the same time.
You will see in this video clip, The Nature of Light from Discovery Education™ streaming, that understanding the dual nature of light was challenging to physicists. Our understanding of light developed over a long period of time, from the time of Isaac Newton, the late 1600’s to the Einstein’s time, the early 1900’s. Today we know that light has a dual nature – it can behave like a particle or a wave. Any experiment using light will illustrate one side of the nature of light but not both at the same time.
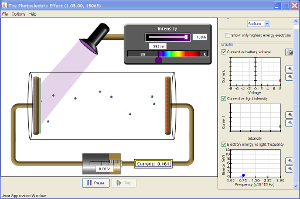
![]() See how the photoelectric effect works by using the PhET simulation, Photoelectric Effect. Begin this simulation by clicking on the image to the right.
See how the photoelectric effect works by using the PhET simulation, Photoelectric Effect. Begin this simulation by clicking on the image to the right.
In the simulation, the metal plate is made of sodium metal. Remember that red light has the lowest frequency and ultraviolet light has the highest, but wavelength is highest for red light and lowest for ultraviolet light. Check the box to create a graph of electron energy versus light frequency. Try to find the threshold frequency for Sodium. Start with the slider at the red end of the spectrum and gradually move it to the left until you see electrons start to be emitted from the Sodium plate. Then adjust the slider to find the largest wavelength that you can have and have no electrons emitted. This color of light represents the threshold frequency.
- You will see that at a wavelength of 540 nm, no electrons are emitted no matter how high you turn up the intensity.
- Now, see what happens if you adjust the wavelength to lower values. Remember that as you reduce wavelength, you are increasing frequency. Observe what happens to the speed of the electrons.
- You should observe that when wavelength is made smaller, the electrons move faster. This happens because each photon is giving more energy to each electron is removes from the sodium atoms. The graph of electron kinetic energy versus frequency should look like one pictured at right. Notice that as the light frequency increases, the electron energy increases steadily.
- With the wavelength at a value where electrons are emitted, observe what happens if you change the intensity.
- You should observe that as the intensity is increased, the number of electrons emitted per unit time increases, but the speed of each electron does not change. This happens because when light is more intense, a larger number of photons are hitting the sodium plate per unit time; however, the energy of each photon is the same.
You will use this simulation again in the application for this topic.
One way to see photons is to look at the absorption and emission of light by a hydrogen atom. The Bohr model of the hydrogen atom explains the origins of the atomic spectrum of hydrogen. Each atom has its own specific spectrum. The colors of light that can be emitted or absorbed are determined by the spacing of the electron energy levels in the atom. The Bohr model of the atom hypothesized that the electron energy levels were fixed; however, Bohr could not explain why the electrons did not spiral toward the nucleus. In the next topic, we will see how another physicist explained the fixed electron orbits.
Dual Nature of Light
 Learn about the dual nature of light by clicking on the steps in this interactivity. Click the player to begin.
Learn about the dual nature of light by clicking on the steps in this interactivity. Click the player to begin.
View a printable version of this interactivity.
Dual Nature of Light Practice
![]()
 Answer each question in the interactivity below, then click submit. Click the player to get started.
Answer each question in the interactivity below, then click submit. Click the player to get started.