Wave-Particle Duality
Answer the following questions. Be sure to show all your work. This activity is available below or in a printable version.
1. Imagine that you are Robert Milliken, a physicist living in the early 1900s when Einstein first proposed the photon theory of light. Based on the wave theory of light, select the hypotheses from the list below that describe how the photoelectric effect experiment should turn out.
a. If the light has enough intensity electrons will be ejected.
b. If the intensity is larger, electrons will have more kinetic energy.
c. Electrons will be emitted for low intensity light if we wait long enough.
d. The light must have a minimum frequency for electrons to be ejected.
e. If light has higher frequency the electrons will have more kinetic energy.
f. Below the threshold frequency, no matter how long you wait, no electrons will be emitted.
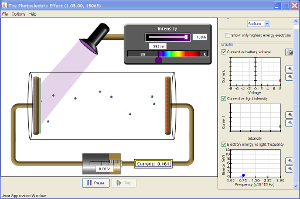
![]() Use the PhET simulation, Photoelectric Effect, to answer questions 2 and 3 below. Click on the image below to start the simulation.
Use the PhET simulation, Photoelectric Effect, to answer questions 2 and 3 below. Click on the image below to start the simulation.
2. Compare two different targets in the PhET simulation. You may select one of the known targets (sodium, zinc, copper, platinum, and calcium), and use the unknown target. Collect electron energy versus light frequency data for each of the three targets. Take screenshots of the graphs for each target.
3. Compare and contrast the three targets. Summarize your findings in a table. Discuss your findings in a paragraph. Your discussion should include:
a. Comparison of the slopes, x-intercepts, and y-intercepts for each graph.
b. Comparison of the work functions and threshold frequencies for each graph.
c. Description the effect of light intensity on electron energy.
4. After Milliken completed the experiment, did his findings support wave theory? Which hypotheses were supported? Justify your answer.
a. If the light has enough intensity electrons will be ejected.
b. If the intensity is larger, electrons will have more kinetic energy.
c. Electrons will be emitted for low intensity light if we wait long enough.
d. The light must have a minimum frequency for electrons to be ejected.
e. If light has higher frequency the electrons will have more kinetic energy.
f. Below the threshold frequency, no matter how long you wait, no electrons will be emitted.
![]()
Once you have finished answering the questions, submit your responses to the dropbox.