Radioactivity
Option A: Radiation In Our World
![]() Research the uses for radioactivity. Create a product (brochure, presentation, or report) that describes how radiation is used in our world. You must include examples for each type of radiation studied in this unit: alpha, beta plus (positron), beta minus (electron), and gamma. Access the Radiation In Our World printable version for complete directions and a checklist for this assignment. Once you have completed this assignment, please submit your work to the dropbox.
Research the uses for radioactivity. Create a product (brochure, presentation, or report) that describes how radiation is used in our world. You must include examples for each type of radiation studied in this unit: alpha, beta plus (positron), beta minus (electron), and gamma. Access the Radiation In Our World printable version for complete directions and a checklist for this assignment. Once you have completed this assignment, please submit your work to the dropbox.
Option B: Nuclear Processes Scientific Investigation
![]() Before you begin the scientific investigation below, make sure to download the Nuclear Processes Scientific Investigation Report. As you complete this scientific investigation, fill in any needed information on the report template. If you need more information about each section of the report, please visit the Developmental Module.
Before you begin the scientific investigation below, make sure to download the Nuclear Processes Scientific Investigation Report. As you complete this scientific investigation, fill in any needed information on the report template. If you need more information about each section of the report, please visit the Developmental Module.
This scientific investigation is available below or in a printable version.
Introduction
Radioactivity is the process by which an unstable nucleus loses energy by emitting particles. Nuclear decay occurs as a result of various nuclear reactions.
Objectives
In this scientific investigation, you will:
- explain radioactive alpha decay.
- explain radioactive beta decay.
- describe how a neutron can give energy to a nucleus and cause it to fission.
- explain how a chain reaction works, and describe the requirements for a sustained chain reaction large enough to make a bomb.
- explain how a nuclear reactor works and how control rods can be used to slow down the reaction.
Hypothesis
Using the three Procedure and Data Collection sections below, read through the procedural information for this scientific investigation. Based on your understanding of the procedure, develop your own hypotheses to verify Conservation of Mass and Energy in Nuclear Reactions. Use the guide questions below as your data/data analysis sections. Utilize actual cost analysis (cite your sources) to determine the most effective and efficient method for production of electrical energy. Record these hypotheses in the Hypothesis section of your Nuclear Processes Scientific Investigation.
Required Simulations
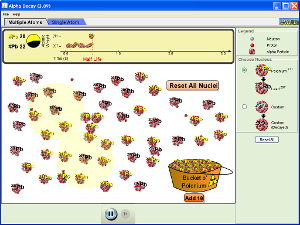
Alpha Decay Simulation
(click on image below to run simulation)
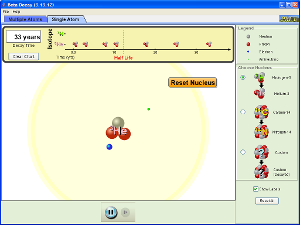
Beta Decay Simulation
(click on image below to run simulation)
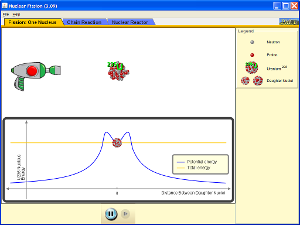
Nuclear Fission Simulation
(click on image below to run simulation)
Provided by:
PhET Interactive Simulations
University of Colorado
http://phet.colorado.edu
Procedure and Data Collection
Alpha Decay Simulation Set-Up
- Open the Alpha Decay simulation.
- Click on the Single Atom tab.
- On the right side of the simulation window, be sure that Polonium-211 nucleus is selected.
Alpha Decay Simulation Procedure and Data Collection
- Observe the decay of Polonium-211. Write a nuclear equation representing the decay of Po-211.
- What happens within the nucleus of Po-211 during decay?
- The half-life of Po-211 is approximately 500 ms or half a second. Below provide the number of undecayed Po-211 atoms at each time interval for a reaction starting with 100 total atoms.
- t=0.5s
- t=1.0s
- t=1.5s
- t=2s
- Now, click on the Multiple Atoms tab of the simulation and simulate the decay of 100 atoms by adding from the "Bucket o' Polonium."
- Compare your predictions in question 3. How would you explain any discrepancies?
Beta Decay Simulation Set-Up
- Open the Beta Decay simulation.
- Click on the Single Atom tab.
- First select the Hydrogen -3 nucleus on the right and observe the beta decay.
- Next select the Carbon-14 nucleus on the right and observe the beta decay.
Beta Decay Simulation Procedure and Data Collection
- Write nuclear equations for the decay processes you just observed.
- Where does the beta particle come from in beta decay? What other particle is produced during the decay process?
Nuclear Fission Simulation Set-Up
- Open the Nuclear Fission simulation.
- Click on the "Fission: One Nucleus" tab.
- Press the red button on the neutron gun and observe the effect on Uranium-235.
Nuclear Fission Simulation Procedure and Data Collection
- Briefly describe how Uranium-235 can be made unstable. Write a nuclear equation to represent this process.
- What do you think would be the effect of firing a neutron into one of 100 atoms of Uranium-235?
- Click on the "Chain Reaction" tab of the simulation.
- Using the slider under U-235 in the lower right corner of the screen, increase the number of atoms to 100.
- Fire the neutron gun and observe.
- Did the results validate your prediction? Explain.
- Based on observations in this simulation, what are the criteria and settings needed to create and atomic bomb?
- Why do you think that "weapons-grade" Uranium would not contain very much Uranium-238?
- Next, click on the Nuclear Reactor tab.
- Run simulations by clicking on the red "Fire Neutrons" button and then moving the green "Control Rod Adjuster."
- What purpose do the control rods serve? Why are they important?
Conclusion
Using the Conclusion section of your Nuclear Processes Scientific Investigation Report, compose three to four sentences describing an overall conclusion about nuclear decay and how it can be harnessed for specific purposes. Were your hypotheses true or false, and how do you know? Use the data and notes that you collected from your simulation experiences to form your conclusion. Make sure that you include information that you gained from data analysis to support your conclusion.
Experimental Sources of Error
On your Nuclear Processes Scientific Investigation Report, provide responses to the following questions: Are there any sources of error? If so, what are they and what could be done to minimize error?
![]() Once you have completed the Nuclear Processes Scientific Investigation, please submit your work to the dropbox.
Once you have completed the Nuclear Processes Scientific Investigation, please submit your work to the dropbox.