
Titrations
A titration is a technique that accurately measures the concentration of an acid or base solution. The technique makes use of a neutralization reaction. Titrations can measure the concentration of a solution because the pH of a neutral solution changes quickly; therefore, the exact concentration can be measured. The process of performing a titration begins with the addition of a known concentrated acid or base called the titrant to a solution of acid or base that is unknown. Since both of the volumes are known, the concentration can be calculated using mathematics.
 Titrations involve carefully adding the titrant to a solution. This is only accomplished through the use of a burette. In this activity, click NEXT to learn about the different laboratory equipment that is used in a titration, and to view how this equipment is setup.
Titrations involve carefully adding the titrant to a solution. This is only accomplished through the use of a burette. In this activity, click NEXT to learn about the different laboratory equipment that is used in a titration, and to view how this equipment is setup.
View a printable version of the interactivity above.
Steps for Completing a Titration
Once you have the laboratory setup completed, you will need to follow a few steps to accurately complete the titration. Take a moment to view the steps required to perform a titration.
- Add a measured volume of an acid of unknown concentration into an Erlenmeyer flask.
- Add several drops of an indicator to the Erlenmeyer flask and mix by swirling the flask.
- Fill a burette with a base solution of known concentration.
- Open the stopcock of the burette, and slowly add the base to the acid while swirling the flask to ensure proper mixing.
- Close the stopcock at the exact moment the indicator changes color.
Titration Curves
 When completing a titration, there are two terms that are identified. The end point is the point when the indicator changes color. This tells you that the acid is neutralized by the titrant and titration is over. During the titration, the number of moles of acid will equal the number of moles of base. This is known as the equivalence point. The equivalence point is different for the three types of titrations. What are the three different titrations? Titrations can exist between strong acids and strong bases, strong acids and weak bases, and weak acids and strong bases. Each of these different titrations can be shown graphically with a titration curve. A titration curve is a graph of pH versus the volume of titrant added. The best way to produce a titration curve is to use a pH meter that will document the change in pH over the course of the titration. In this interactivity, click on the images in the media panel to investigate the types of titrations by analyzing their titration curves.
When completing a titration, there are two terms that are identified. The end point is the point when the indicator changes color. This tells you that the acid is neutralized by the titrant and titration is over. During the titration, the number of moles of acid will equal the number of moles of base. This is known as the equivalence point. The equivalence point is different for the three types of titrations. What are the three different titrations? Titrations can exist between strong acids and strong bases, strong acids and weak bases, and weak acids and strong bases. Each of these different titrations can be shown graphically with a titration curve. A titration curve is a graph of pH versus the volume of titrant added. The best way to produce a titration curve is to use a pH meter that will document the change in pH over the course of the titration. In this interactivity, click on the images in the media panel to investigate the types of titrations by analyzing their titration curves.
View a printable version on the interactivity.
Solving a Titration using Mathematics
Once you have completed your titration, you have determined the volume of the acid and base. You already knew the molarity of the known titrant. You will need to use mathematics to discover the molarity of the solution in the Erlenmeyer flask. To do this you will need to use a formula.
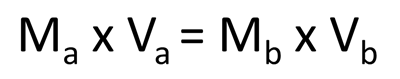
 In this equation, Ma is equal to the molarity of the acid and Va is equal to the volume of the acid. Mb represents the molarity of the base and Vb is equal to the volume of the base. You can rearrange this equation to solve for any of the unknown variables in a titration. In this activity, click on the numbered steps to learn how an example titration problem is solved.
In this equation, Ma is equal to the molarity of the acid and Va is equal to the volume of the acid. Mb represents the molarity of the base and Vb is equal to the volume of the base. You can rearrange this equation to solve for any of the unknown variables in a titration. In this activity, click on the numbered steps to learn how an example titration problem is solved.
View a printable version of the interactivity below.
Titrations Review

![]() Now that you have learned how to complete a titration and solve problems related to titrations, complete this non-graded activity to check your knowledge. Read each question, identify the known and unknown variables, and use the titration equation to solve the problem. Then, select the appropriate answer and click SUBMIT to check your response. Click on the interactivity thumbnail, and then click NEXT to get started.
Now that you have learned how to complete a titration and solve problems related to titrations, complete this non-graded activity to check your knowledge. Read each question, identify the known and unknown variables, and use the titration equation to solve the problem. Then, select the appropriate answer and click SUBMIT to check your response. Click on the interactivity thumbnail, and then click NEXT to get started.


