
The Quantum Mechanical Model
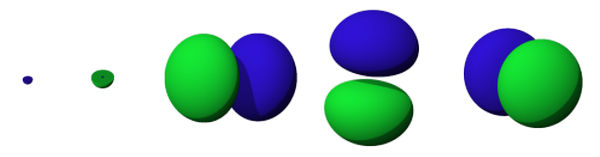
The shapes of the first five atomic orbitals: 1s, 2s, 2px, 2py, and 2pz
Electrons do not reside on perfectly shaped rings outside the nucleus, as the Bohr model suggested. Instead, the electrons are moving around in electron clouds, or regions of high electron probabilities. You cannot see what these clouds look like, but based on the mathematics of the quantum mechanical model, you can determine the probable locations of an electron. These regions, called orbitals, take on various shapes and sizes. The maximum number of electrons in any one orbital depends on that orbital's location outside of the nucleus. These orbitals are diagrammed and organized in charts called orbital diagrams.




