Molecular Polarity
 Molecular Polarity Scientific Investigation
Molecular Polarity Scientific Investigation
![]() Before you begin the scientific investigation below, make sure to download the Molecular Polarity Investigation Report. As you complete this scientific investigation, fill in any needed information on the report template. If you need more information about each section of the report, please visit the Developmental Module.
Before you begin the scientific investigation below, make sure to download the Molecular Polarity Investigation Report. As you complete this scientific investigation, fill in any needed information on the report template. If you need more information about each section of the report, please visit the Developmental Module.
This scientific investigation is available below or in a printable version.
Introduction
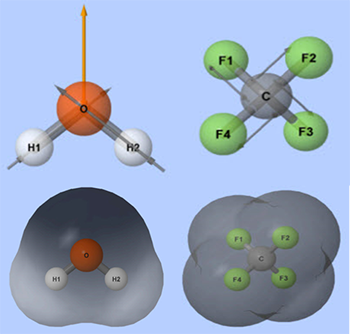
Molecular polarity is a measure of how electrons are distributed in the outer regions of the molecule. It is similar to bond polarity in that it is a measure of how unequally electrons are shared. It is different than bond polarity because bond polarity is a measure of how unequally the electrons are shared in a bond, while molecular polarity is a measure of how unequally the electrons are distributed throughout the outer region of the entire molecule, not just a particular bond. Molecular polarity is the sum of all the bond polarities. A highly polar molecule will have an uneven distribution of electrons around the outer regions of the molecule, which will result in areas of positive and negative charges. A non-polar molecule has the electrons uniformly distributed around the outer edges. Non-polar molecules have no areas of positive or negative charges in the outer region of the molecule. The above shows water with two highly polar O-H bonds. The bent shape causes an uneven distribution of electrons. Also, the image above shows carbon tetrafluoride. Carbon tetrafluoride has four highly polar C-F bonds that are arranged in opposite directions so they cancel each other out. In carbon tetrafluoride, the electrons are uniform throughout the molecule.
Objectives
In this scientific investigation, you will:
- explore the relationships between bonds and atoms, electron location, and the interaction of molecules to view the effects on molecular polarity; and
- determine how electronegativity and molecular shape determine the polarity of a molecule.
Hypothesis
Using the Procedure and Data Collection section below, read through the procedural information for this scientific investigation. Based on your understanding of the procedure, develop your own hypotheses which describe the expected results. You should consider the following questions: How does electronegativity affect the polarity of a molecule? How does molecular shape affect molecular dipole? Record these hypotheses in the Hypothesis section of your Molecular Polarity Scientific Investigation Report.
Required Simulation
Molecular Polarity
(click on image below to run simulation)
Provided by:
PhET Interactive Simulations
University of Colorado
http://phet.colorado.edu
Part I
Procedure and Data Collection
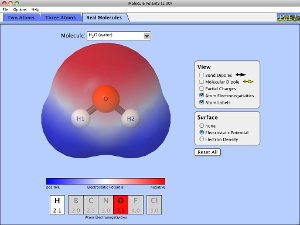
 Simulation Set-Up (see image at right)
Simulation Set-Up (see image at right)
- Open the Molecular Polarity simulation.
- In the top tab, make sure that the Three Atoms tab is selected.
- In the View box, click the checkboxes for bond dipole, molecular dipole, and partial charges.
- In the Electric Field box, click the checkbox for off.
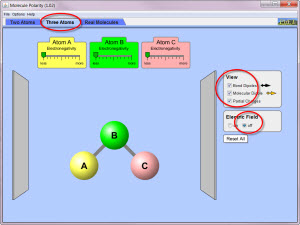
Molecular Polarity with Three Atoms
- Run the Simulation with different combinations of electronegativities for atoms A, B, and C, as specified in the data table below. Use the key below to help you run the simulation.
- Electronegativity Settings: less, middle, more
- Bond Diploe and Molecular Dipole Strength: zero, medium, and strong
- Direction: north, south, east, west, northeast, northwest, southeast, and southwest.
- Observe the size and direction of the dipoles and partial charges. Record your observations in the data table.
Data
Use the data table provided on your Molecular Polarity Scientific Investigation Report to record your data from this scientific investigation. The data table is also shown below:
| Electronegativity Settings | A-B Dipole | C-B Dipole | Molecule Dipole | |||||
|---|---|---|---|---|---|---|---|---|
| Less | Less | Less | ||||||
| Less | Less | Middle | ||||||
| Less | Less | More | ||||||
| Less | Middle | More | ||||||
| Less | Middle | Less | ||||||
| Less | Middle | Middle | ||||||
| Less | Middle | More | ||||||
| Middle | Less | Less | ||||||
| Middle | Less | Middle | ||||||
| Middle | Less | More | ||||||
| Middle | Middle | Less | ||||||
| Middle | Middle | Middle | ||||||
| Middle | Middle | More | ||||||
| Middle | More | Less | ||||||
| Middle | More | Less | ||||||
| Middle | More | More | ||||||
| More | Middle | Less | ||||||
| More | Middle | Middle | ||||||
| More | Middle | More | ||||||
Data Analysis
In the Data Analysis section of your Molecular Polarity Scientific Investigation Report, provide the responses to the following questions:
- What combinations of electronegativities created a non-polar, polar, and highly polar molecule?
- How do the changes in the strength of the individual bond dipoles affect the strength and direction of the molecular dipole?
- What is the relationship between the molecular dipole and the individual bond dipoles?
Part II
Procedure and Data Collection
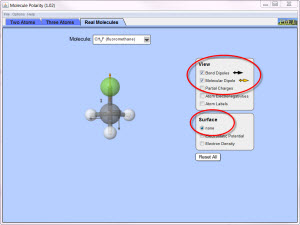 Simulation Set-Up (see image at right)
Simulation Set-Up (see image at right)
- Open the Molecular Polarity simulation.
- In the top tab, make sure that the Real Molecules tab is selected.
- In the View box, click the checkboxes for bond dipole and molecular dipole.
- In the Surface box, click the checkbox for none.
Molecular Polarity with Real Atoms
- Select five molecules that have the following shapes: linear, bent, trigonal pyramidal, and tetrahedral.
- Complete the table below for each of the five different molecules.
Data
Use the data table provided on your Molecular Polarity Scientific Investigation Report to record your data from this scientific investigation. The data table is also shown below:
Molecular Formula |
Lewis Dot Structure |
Molecular Shape |
Sketch of the Molecule with Bond Dipoles and Molecular Dipoles |
|---|---|---|---|
Data Analysis
In the Data Analysis section of you Molecular Polarity Scientific Investigation Report, provide responses to the following questions:
- Is it possible to have a polar molecule that is made up of non-polar bonds? Explain your answer.
- Is it possible to have a polar molecule that is made up of polar bonds? Explain your answer.
Conclusion
Using the Conclusion section of your Molecular Polarity Scientific Investigation Report, compose three to four sentences describing an overall conclusion about the relationship between electronegativity, molecule shape, and polarity. Base your conclusions on your data. Were your hypothesis true or false, and how do you know? Use the data and notes that you collected from your simulation experience to form your conclusion. Make sure that you include information that your gained from data analysis to support your conclusion.
Experimental Sources of Error
On your Molecular Polarity Scientific Investigation Report, provide responses to the following questions: Are there any sources of error? If so, what are they, and what could be done to minimize error?
![]() Once you have completed the Molecular Polarity Scientific Investigation Report, please submit your work to the dropbox.
Once you have completed the Molecular Polarity Scientific Investigation Report, please submit your work to the dropbox.