
Molecular Polarity
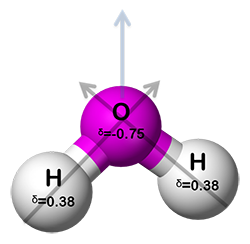
A water molecule
Molecules can be classified as polar or nonpolar. You learned it is possible for a molecule to be nonpolar when it has polar bonds, if the bonds are arranged symmetrically around the central atom. It is also possible for a molecule to be nonpolar, if all of its bonds are nonpolar. In this situation, the electrons in the molecule are distributed and shared equally.
In a polar molecule, the electrons tend to be on one side of the molecule more than another because the bond polarities do not cancel out. This makes the molecule like a magnet, with one side positive and the other side negative. You are probably familiar with the polar effect of magnets. Linear, tetrahedral, and trigonal planar shapes have the possibility of being polar or nonpolar. Bent and pyramid shapes are always polar molecules because they both have unshared electrons around a central atom.




