
Quantum Mechanics
Answer the following questions. Be sure to show all your work. This activity is available below or in a printable version.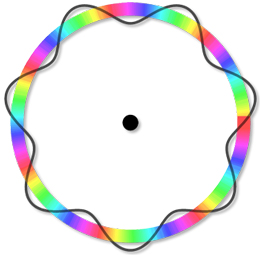
Multiple choice. Indicate the best answer.
1. How did DeBroglie explain the fixed electron orbitals in the Bohr Model?
a. Using Quantum Mechanics
b. Through the Heisenberg Uncertainty Principle
c. Using standing waves
d. None these
2. According to DeBroglie’s hypothesis, the wavelength of a particle depends on the
a. momentum of the particle
b. mass of the particle
c. diameter of the particle
d. none of these
3. The importance of the wave nature of particles was that
a. Particles were always moving
b. Particles have color
c. Particles could interfere just like waves
d. It was not important
4. Which of the following devices uses the wave nature of particles?
a. Solar cell
b. Electron microscope
c. Nuclear reactor
d. None of these
5. The Heisenberg uncertainty principle is
a. The more precisely we know position, the less precisely we can know the momentum of a particle.
b. The more precisely we know position, the more precisely we can know the momentum of a particle.
c. The less precisely we know position, the less precisely we can know the momentum of a particle.
d. None of these
![]()
Once you have finished answering the questions, submit your responses to the dropbox.



